Figure 2.
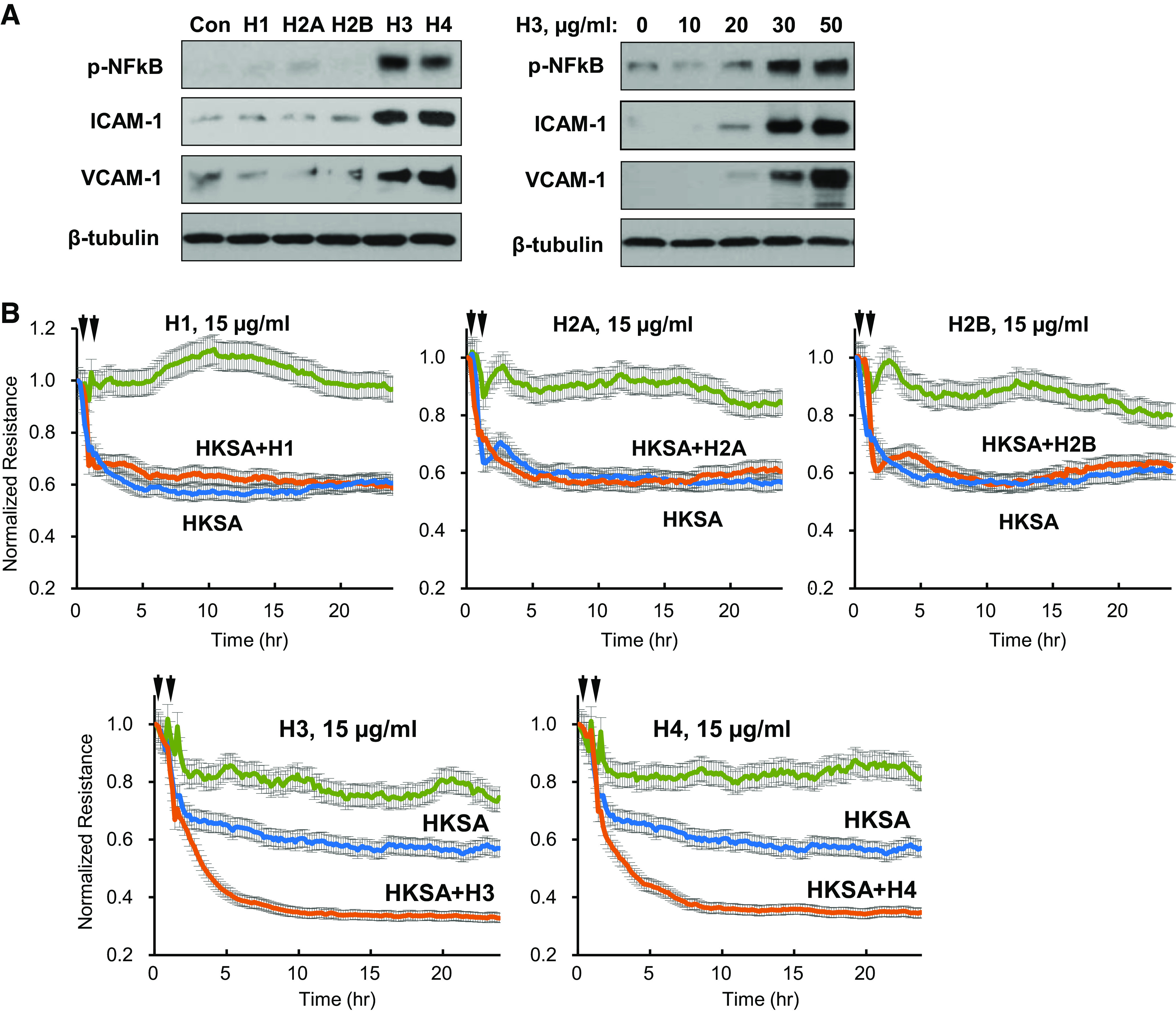
Histones H3 and H4 augment agonist-induced endothelial barrier disruption. A: HPAECs were treated with 50 µg/mL of each histone subunit. Expression of indicated inflammatory proteins was detected by Western blotting (left). Dose-dependent effect of histone H3 on expression of inflammatory markers (right). Shown are representative blots from four independent experiments. B: TER measurements in HPAEC monolayers treated with HKSA alone (5 × 108 particles/mL) or with addition of indicated histone subtypes (15 µg/mL, added 30 min after HKSA stimulation). Single stimulation with HKSA or each histone subunit was used as comparison controls. Shown are averaged TER traces from 8 to 10 individual runs. C: at the time points marked by first arrows, EC was treated with vehicle, heat-killed bacteria (HKSA, HKEC, HKSP, HKPA; 5 × 108 particles/mL), FSL1 (200 ng/mL), CRX-527 (100 ng/mL), TNFα (5 ng/mL), or LPS (100 ng/mL) followed by stimulation with H3 subunit (20 µg/mL, second arrows). TER was monitored over 20–24 h. Bar graph summarizes TER changes in HPAEC monolayers 10 h after agonist stimulation; *P < 0.05, n = 5–8. D: cells were exposed to LPS, TNFα, or heat-killed bacteria: HKSA, HKSP, HKEC, or HKPA alone or in combination with H3 (20 µg/mL). Visualization of permeability for FITC avidin was performed as described in materials and methods. FITC fluorescence images depict FITC-avidin tracer added to culture medium and bound to biotin-coated matrix. Counterstaining of cell nuclei was performed by DAPI. Images were randomly selected from 10 to 12 microscopic fields/condition from three independent experiments: bar = 10 µm. Bar graph depicts quantitative analysis of tracer fluorescence intensity; *P < 0.05. HKEC, heat-killed E. coli; HKPA, heat-killed P. aeruginosa; HKSA, heat-killed S. aureus; HPAEC, human pulmonary artery endothelial cell; LPS, lipopolysaccharide; TER, transendothelial electrical resistance; TNF-α, tumor necrosis factor-α.
