Figure 5.
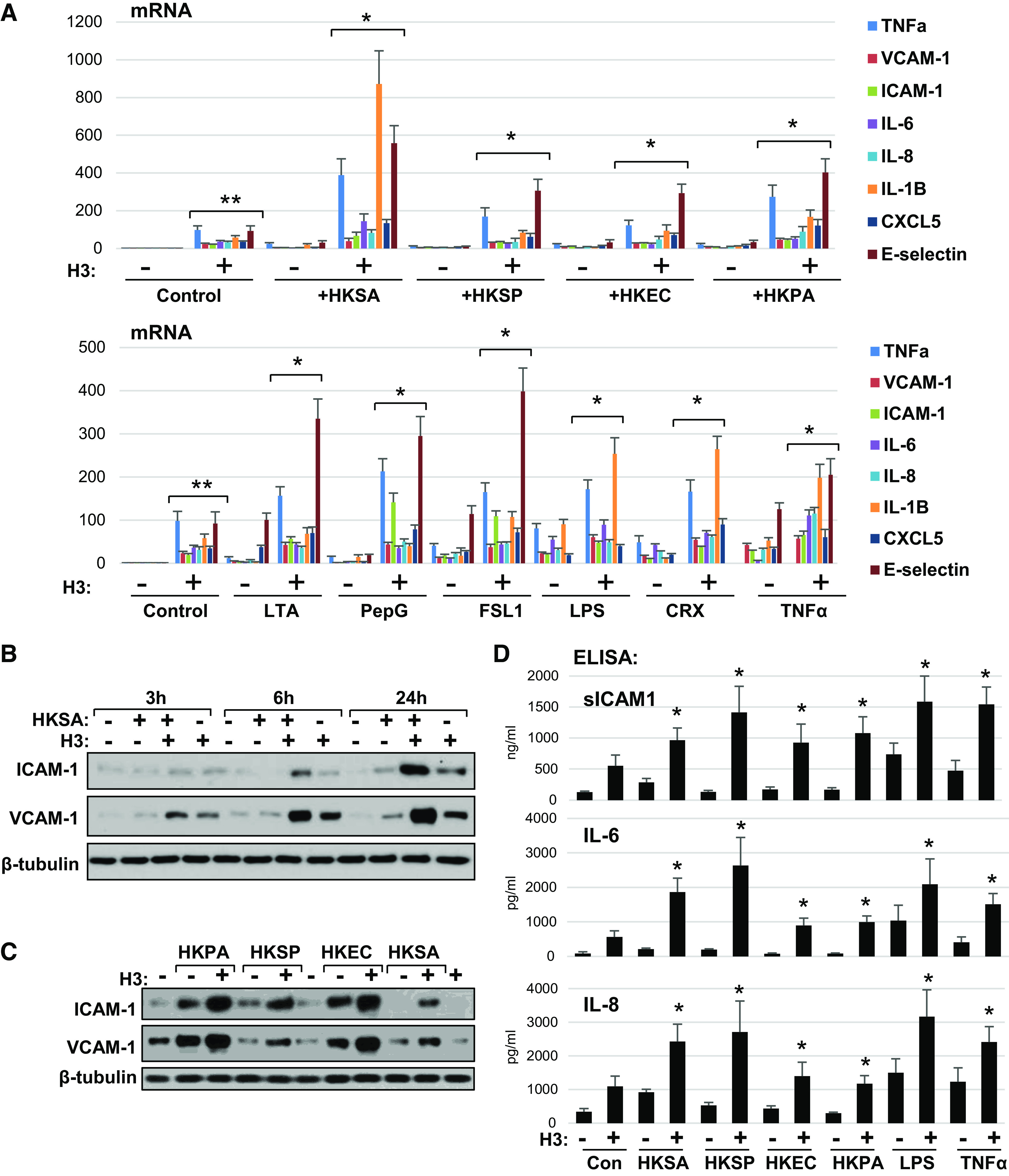
Universal augmenting effect of H3 histone on endothelial inflammation caused by various pathogens. A: HPAEC exposed to indicated heat-killed bacteria (5 × 108 particles/mL) with or without H3 (20 µg/mL) for 3 h were subjected for qPCR analysis. Bar graph depicts changes in expression of mRNA transcripts of various proinflammatory cytokines, chemokines, and adhesion molecules. Data represent means ± SD, n = 5, **P < 0.01 vs. vehicle; *P < 0.01 vs. agonist alone. Bottom: similar qPCR analysis of EC stimulated with bacterial cell wall components: LTA (200 ng/mL), PepG (200 ng/mL), LPS (25 ng/mL); specific TLR2/6 (FSL1, 200 ng/mL) and TLR4 (CRX-527, 25 ng/mL) activators, or TNFα (0.1 ng/mL) alone or in combination with 20 µg/mL H3. B: HKSA and histone H3 were added to HPAEC either alone or in combination and incubated for indicated times followed by Western blot analysis of ICAM-1 and VCAM-1 protein expression levels. C: HPAECs were treated with indicated heat-killed bacteria alone or in combination with histone H3, and immunoblotting was performed to detect ICAM-1 and VCAM-1 protein levels. Shown (B and C) are Western blot results representative of three independent experiments. D: secretion of sICAM-1, IL-6, and IL-8 was determined by ELISA of conditioned media obtained from cells treated with H3 in combination with other agonists as in A. Data are means ± SD, n = 3, *P < 0.05 vs. stimulation with indicated agonist alone. HPAEC, human pulmonary artery endothelial cell; HKSA, heat-killed S. aureus; LPS, lipopolysaccharide; LTA, lipoteichoic acid; PepG, peptidoglycan-G; TNF-α, tumor necrosis factor-α.
