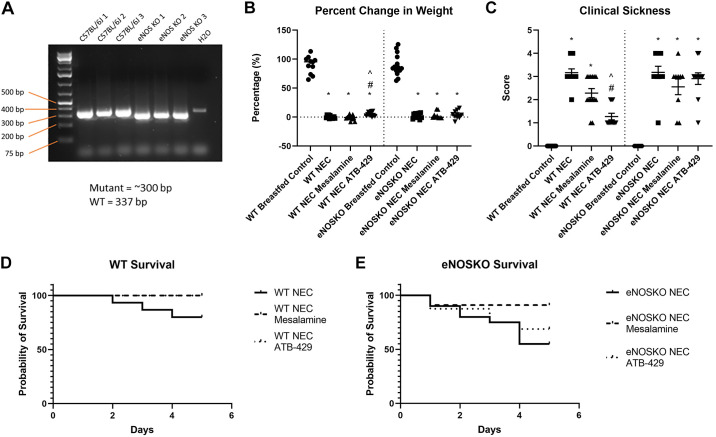
Figure 1.
ATB-429 decreases weight loss, clinical sickness score, and mortality in experimental NEC. Experimental NEC was performed by subjecting neonatal mice to hypoxia, hypothermia, enteral LPS, and formula feeding. Survival and weight loss were monitored, and clinical sickness score was recorded as described in methods. A: mutant eNOSKO mice purchased from Jackson Laboratories were validated using PCR. B: ATB-429 significantly attenuated weight loss in experimental NEC in WT mice but did not affect weight loss in eNOSKO mice. C: ATB-429 improved clinical sickness score in WT mice but had no effect in eNOSKO mice. D: survival curves demonstrating a benefit for both mesalamine and ATB-429 in WT mice, P = 0.0407. E: when the experiment was repeated in eNOSKO mice, there was no survival benefit for mesalamine or ATB-429, P = 0.7787. Statistical analysis for weight loss and clinical sickness was performed using ANOVA with Tukey’s multiple-comparisons test when parametric and Kruskal–Wallis with Dunn’s multiple-comparisons test when nonparametric. Log-rank (Mantel–Cox) test was performed for survival curves. Total n: WT Breastfed Control, n = 11; WT NEC, n = 15; WT NEC Mesalamine, n = 14; WT NEC ATB-429, n = 12; eNOSKO Breastfed Control, n = 15; eNOSKO NEC, n = 20; eNOSKO NEC Mesalamine = 11; eNOSKO NEC ATB-429 = 16. *P < 0.05 vs. Breastfed Control, #P < 0.05 vs. NEC, ^P < 0.05 vs. NEC Mesalamine. eNOSKO, endothelial nitric oxide synthase knockout mice; LPS, lipopolysaccharide; NEC, necrotizing enterocolitis; WT, wild type. n, number of mice.