Abstract
Context
Sarcopenia is a prevalent syndrome that has seen increased awareness in the last twenty years.
Objective
To systematically assess and evaluate the utility of bioelectrical impedance analysis (BIA) in the diagnosis of sarcopenia in adults over the age of 60.
Methods
An electronic search strategy of databases was conducted, including Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, and SportDiscus. Included articles were evaluated using The Quality Appraisal for Reliability Studies (QAREL) checklist.
Results
Seven articles (1336 participants) met the inclusion criteria of evaluating the diagnostic ability of BIA. Results indicate that that there is a high degree of heterogeneity in how BIA is used to diagnose sarcopenia. While BIA is an affordable and easy to use measurement tool, it does not consistently demonstrate high levels of diagnostic sensitivity.
Conclusion
The current evidence does not consistently support the utility of BIA as an accurate diagnostic tool for sarcopenia in adults over 60. If utilizing BIA, clinicians should select a validated BIA equation for their patient’s demographics. Clinicians should also consider the use of functional tests and validated screening questionnaires.
This systematic review was registered at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=211586
Keywords: aging, bioelectrical impedance, diagnosis, sarcopenia, screening, systematic review
MOTS CLÉS: dépistage, diagnostic, examen systématique, impédance bioélectrique, sarcopénie, vieillissement
Abstract
Contexte
La sarcopénie est un syndrome répandu qui a suscité une attention accrue ces vingt dernières années.
Objectif
Examiner et évaluer systématiquement l’utilité d’une analyse d’impédance bioélectrique dans le diagnostic de la sarcopénie chez des adultes âgés de plus de 60 ans.
Méthodologie
Une stratégie de recherche électronique sur des bases de données a été appliquée, notamment le Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL et SportDiscus. Les articles en question ont été évalués grâce à la liste de vérification de l’instrument Quality Appraisal for Reliability Studies (QAREL).
Résultats
Sept articles (1 336 participants) remplissaient les critères d’inclusion de l’évaluation de la capacité diagnostique de l’analyse d’impédance bioélectrique. Les résultats montrent un degré élevé d’hétérogénéité dans l’utilisation de l’analyse d’impédance bioélectrique pour diagnostiquer la sarcopénie. Si l’analyse d’impédance bioélectrique est un outil de mesure fiable et facile à utiliser, il ne démontre pas systématiquement de hauts niveaux de sensibilité diagnostique.
Conclusion
Les données probantes actuelles ne justifient pas systématiquement l’utilité d’une analyse d’impédance bioélectrique comme outil diagnostic précis de la sarcopénie chez des adultes âgés de plus de 60 ans. S’ils utilisent l’analyse d’impédance bioélectrique, les cliniciens doivent choisir une équation de l’analyse validée pour les données démographiques de leurs patients. Les cliniciens doivent également envisager de recourir à des examens fonctionnels et à des questionnaires de dépistage validés.
L’examen systématique a été consigné sur le site https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=211586
Introduction
Sarcopenia, defined as poverty or deficiency of flesh, was first described by Rosenberg in 1989.1 Sarcopenia is a degenerative muscular disease in which individuals have decreased muscle quantity, quality, and strength.2,3 Between the ages of 20 and 80 years old, the average adult experiences a reduction of roughly 30% of their muscle mass and a decline in cross-sectional area of approximately 20%.4 To better address the needs of individuals with sarcopenia, multiple international working groups targeting sarcopenia have been created, each with their own operational definition for the condition.2,5–7 While differences exist between these definitions, all acknowledge that sarcopenia is characterized by a decrease in muscle strength and function. Sarcopenia, in this regard, is viewed as the presence of both low muscle mass and low muscle function. Reported prevalence estimates for sarcopenia in community-dwelling older adults (>60 years) vary between 1% and 52% based on the definition and measurements used.8
Multiple methods exist to measure muscle mass or function for the diagnosis of sarcopenia.8 Although magnetic resonance imaging (MRI) is considered the reference standard for measuring lean body mass, it is costly and not readily available.8,9 Several clinically based measures have also been used to detect sarcopenia. These include tests such as bioelectrical impedance analysis (BIA), grip strength via handheld dynamometry, gait speed, the short physical performance battery, SARC-F functional test, the sarcopenia – Quality of Life (SarQoL) questionnaire, and the Short Portable Sarcopenia Measure (SPSM).2,9–11 Given the aging population and complications related to sarcopenia, it is critically important to find practical and affordable measures to diagnose the condition.
Bioelectrical impedance analysis was originally designed as a tool to measure total body water and fat free mass.10 BIA offers a fast, affordable, easy-to-use method for the estimation of lean body mass by clinicians, including sports medicine physicians, physiotherapists, and chiropractors.11 With BIA, lean body mass is measured using electrical conductance to determine the resistance to flow as the current passes through the body, without the use of radiation. This provides estimates of body composition using specific equations programmed into the device, which can assist in the detection of low muscle mass.11–13 BIA has been studied in a variety of populations, demonstrating its popularity as a measure of lean body mass worldwide. However, previous reviews have not explicitly evaluated the diagnostic utility of BIA in adults over the age of 60 years old.
The purpose of this systematic review was to identify and critically appraise the utility of bioelectrical impedance analysis to measure lean body mass in order to detect sarcopenia, and to determine its diagnostic validity in a clinical setting. In this study, we present findings related to the diagnostic utility of BIA for sarcopenia in adults over 60 years old.
Methods
Search strategy
A search strategy was developed in consultation with a health sciences librarian with the search performed on 28 April 2020.14 The basic search strategy was developed using key search terms such as, “Sarcopenia” OR “lean body mass” AND “bioelectrical impedance analysis” AND “DXA” AND “Validity” OR “Reliability.” Search terms consisted of subject headings specific to each database (e.g., MeSH in MEDLINE) and free text words relevant to sarcopenia measures, validity, and reliability (Appendix I).
The following electronic databases were systematically searched from database inception to April 2020: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, and SportDiscus. The systematic review search strategy was similar to the strategy used previously by Shafiee et al.15 on a similar topic to confirm that relevant studies were found.
Selection of studies
To be included in the systematic review, studies had to fulfill the following inclusion criteria: 1) English language; 2) published in a peer-reviewed journal; 3) study designs included observational studies including cohort studies, cross-sectional studies, case series and randomized controlled trials if they met other inclusion criteria; 4) study population adults 60 or older (study could report on other ages, but the ability to extract data for participants 60+ years old had to be available for inclusion); 5) measures included bioelectrical impedance analysis (BIA) compared to ultrasound sonography (USS), dual-energy x-ray absorptiometry (DXA), computerized tomography (CT) or magnetic resonance imaging (MRI) 6) measuring lean muscle mass or diagnosing sarcopenia; 7) statistical measures included at least one of the following: sensitivity, specificity, positive and negative predictive values, reliability, validity, limits of agreement, mean difference, standard error of estimates and responsiveness of BIA as measure of lean muscle mass or sarcopenia. For the synthesis presented in this manuscript, only studies that utilized BIA to diagnose sarcopenia were included.
Studies fulfilling any of the following criteria were excluded: 1) publication types including: guidelines, letters, editorials, commentaries, unpublished manuscripts, dissertations, government reports, books and book chapters, conference proceedings, meeting abstracts, lectures and addresses, consensus development statements; 2) study designs including: pilot studies, case reports, qualitative studies, non-systematic and systematic reviews, clinical practice guidelines, biomechanical studies, laboratory studies, studies not reporting on methodology; 3) cadaveric or animal studies; 4) tests or measures which do not include a measure of BIA; 5) sarcopenia muscle mass tests which did not compare to bioelectrical impedance analysis.
Screening of titles and abstracts
All potentially relevant citations identified by the search strategy were exported into EndNote X6 for reference management, study screening, and removal of duplicates. Rotating pairs of trained reviewers independently screened articles in two phases using a standardized pre-piloted Excel spreadsheet. The first phase involved screening titles and abstracts for relevance based on criteria described above, with potential ratings of relevant, possibly relevant, and irrelevant. At the end of phase one screening, disagreements between reviewers were resolved by discussion to reach consensus. In the second phase, a single pair of reviewers screened the full text articles of the possibly relevant citations to determine whether they were relevant to be included.
Critical appraisal and data extraction
All relevant studies were critically appraised by a pair of reviewers. The Quality Appraisal for Reliability Studies (QAREL) checklist was used to critically appraise the quality of the studies included.16 Consensus between reviewers was reached through discussion. The single pair of reviewers extracted data from eligible studies to build evidence tables and a second review was done to confirm the study results. Data extracted from each study included author, publication year, study design, setting and participants (age, sex, number), assessment tool, reference standard used, outcome measured, sensitivity and specificity, negative and positive predictive value, negative and positive likelihood ratio, and inter-rater and intra-rater reliability. The QAREL checklist allowed for methodological quality to be assessed but did not lead to the exclusion of any studies.
Statistical analyses
We computed the inter-rater reliability for the screening of titles and abstracts using percent agreement and kappa coefficients (κ) with 95% confidence intervals (CI) for phase 1. We used data provided in the studies and computed the 95% CI where information was available. We reported sensitivity, specificity, positive and negative predictive values with 95% CI.
Results
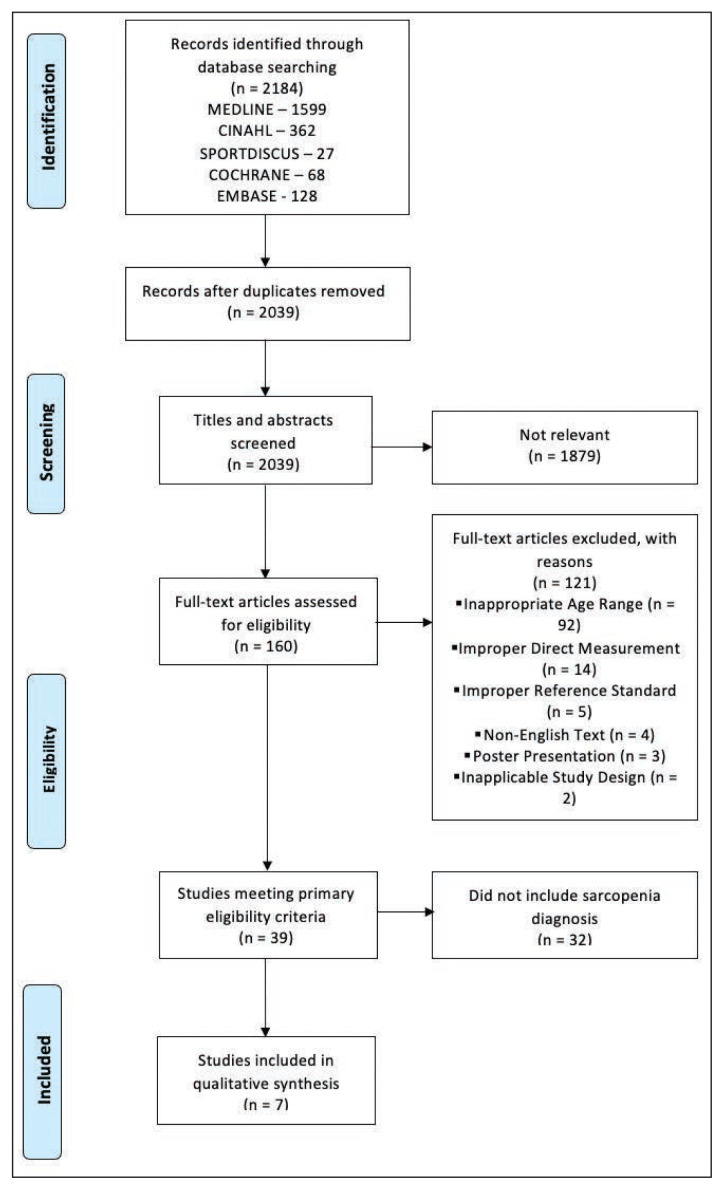
Figure 1 displays the flow of articles identified through the search strategy through the screening phases. The initial search yielded 2184 potentially relevant titles and abstracts from five different databases, with 2039 remaining after duplicates were removed. Upon phase one of title and abstract screening, 1879 were found not relevant. Full-text articles (160) were reviewed with 121 being excluded as not relevant. Thirty-nine studies were further assessed for statistical analysis and purpose, with 32 excluded from the synthesis presented here as they did not assess the ability to diagnose sarcopenia. Finally, seven studies reporting on BIA as part of a diagnostic tool for sarcopenia were included in this review.
Figure 1.
PRISMA flow diagram
Screening
For phase 1 title and abstract screening, screeners 1 and 2 screened 691 titles and abstracts with % agreement 87.0 (95%CI 84.5–89.5) and kappa 0.30 (95%CI 0.20–0.40), screeners 1 and 3 screened 669 title and abstracts with % agreement 93.6 (95%CI 91.7–95.4) and kappa 0.63 (95%CI 0.53–0.73), and screeners 1 and 4 screened 672 titles and abstracts with % agreement 96.9 (95%CI 95.6–98.2) and kappa 0.62 (95%CI 0.47–0.77). Phase 2 involved a single person assessing the full text with a second individual reviewing afterward to verify. The majority of studies were excluded as participants were in the inappropriate age range.
Study characteristics
Seven studies assessed BIA as a tool alone or in combination with other measurements for the diagnosis of sarcopenia, of which two also assessed the test-retest of BIA.17–23 Six studies used Dual-energy X-ray Absorptiometry (DXA), and one study used ultrasound sonography (USS) as reference standard comparators. A total of four different body composition measurements were described, with appendicular skeletal muscle mass (ASMM) being used five times18,20–23, and appendicular muscle mass17 and fat-free mass19 being used once each.
The number of participants included in the studies ranged from 60 to 438, with a total of 1336 participants (472 males and 870 females). The participants ranged in age from 65 to 98 years old. The studies included community-dwelling or free-living individuals, and in-patients. The geographic location varied among studies as they took place in six different countries with two occurring in Austria21,23, and one study occurring in the United States20, Japan17, Indonesia18, Taipei19, and Norway22.
Methodological critical appraisal of included studies
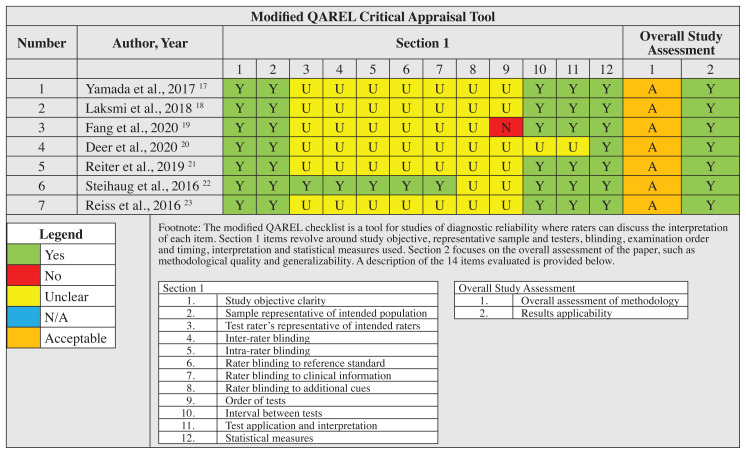
All seven studies demonstrated acceptable methodological quality based on appropriate study design for the assessment of muscle mass in older adults. However, studies did not consistently include information regarding the individuals performing the measures, or pre-measurement factors such as fasting or fed state of the participants (Table 1). Studies were not excluded due to sample size at any point during this systematic review.
Table 1.
Modified QAREL critical appraisal tool study assessment
Fasting protocol
Only one study described the fasting protocol used for participants while the other six studies did not state if a fasting versus non-fasting protocol was used. Fang et al.19 stated that all subjects did not consume alcohol for at least 48 hours, perform vigorous exercise for at least 12 hours, or consume a meal or drink for at least 12 hours prior to the examination. No study compared fasting versus nonfasting in this population to determine its importance in BIA measurement utility. No conclusion can be made at this time regarding the utility of fasting or fasting protocols for accuracy of BIA measure as part of the diagnosis of sarcopenia.
Types of BIA measures
Four studies used single-frequency BIA (SF-BIA)20–23 and three studies used multi-frequency BIA (MF-BIA).17–19 One study used the InBody 720 which has its own proprietary formula.19 The type of BIA used as part of the diagnosis of sarcopenia may be significant, as measurements made by different frequency devices have shown different levels of accuracy in specific populations.10 However, no conclusions on the superiority of one type of BIA measurement can be made in this review as no studies compared MF-BIA to SF-BIA for the detection of sarcopenia.
Sarcopenia definitions
Several definitions for sarcopenia were utilized. The European Working Group on Sarcopenia in Older People (EWGSOP), the Janssen cut-off, and the Chien cut-offs were each used in one study, while the Asian Working Group for Sarcopenia (AWGS) definition, the Foundation for the National Institutes of Health (FNIH) definition, and Baumgartner’s definition was used in two studies. Each definition had a differing cut-off point for the diagnosis of sarcopenia. It is relevant to note that no global consensus definition exists for muscle mass cut offs as the diagnosis of sarcopenia varies based on population characteristics, including anthropometric and lifestyle differences.7
Diagnostic ability of sarcopenia
Seven studies using a total of 13 predictive equations (10 unique) assessed the ability of BIA alone, or in combination with other variables, to diagnose sarcopenia (Table 2). As some studies used multiple cut points and equations, a total of 23 unique combinations of patient, equation, and cut points were extracted. Inconsistent findings were seen in the various studies when BIA was used in the diagnosis of sarcopenia. The majority of the formulas that included BIA to diagnose sarcopenia also incorporated factors such as age, weight, and height, in addition to impedance as measured by BIA. Therefore, BIA is often used in combination with other variables in formulas to predict muscle mass for the diagnosis of sarcopenia, rather than being used as a stand alone determinant.
Table 2.
Data extraction from seven studies including description of studies and the diagnostic properties of BIA based diagnostic tools for diagnosis of sarcopenia
| Study author, year | Setting, Population | BIA Device | Equation | Sarcopenia Muscle Mass Cut-Offs | Subgroup | Sample Size | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | + Likelihood Ratio | − Likelihood Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamada 2017 17 | Community dwelling older adults 65yr +, Tokyo Japan | MSd-100 (Tanita Corp., Tokyo, Japan) | Tanita device proprietary equation – unspecified | Post Hoc Cutoffs < 7.18 kg/m2 |
men | N=100 | 0.66 (0.55, 0.79) | 0.57 (0.42, 0.73) | 0.70 (0.58, 0.82) | 0.53 (0.39, 0.68) | 1.53 | 0.60 |
| < 5.89 kg/m2 | women | N=247 | 0.62 (0.53, 0.70) | 0.60 (0.51, 0.68) | 0.60 (0.52, 0.69) | 0.61 (0.52, 0.70) | 1.55 | 0.63 | ||||
| Laksmi 2018 18 | Consecutive patients 60yr + Geriatric Clinic Jakarta Indonesia | BIA Tanita MC-780MA (Tokyo, Jepang) | Tanita device proprietary equation – unspecified | AWGS:* Men: < 7.0 kg/m2 Women: < 5.7kg/m2 |
all | N=120 | 0.79 | 0.67 | 0.37 | 0.93 | 2.38 | 0.31 |
| Fang 2020 19 | Elderly patients undergoing health examination, Taipei, Taiwan | InBody 720; Biospace | InBody proprietary equation – unspecified | AWGS:* Men: < 7.0 kg/m2 Women: < 5.4kg/m2 |
Validation sample n=84 for sensitivity & specificity | N=438 (N=84 for sensitivity & specificity) | 0.66 | 1.00 | ∞ | 0.34 | ||
| Deer 2020 20 | Inpatients University of Texas hospital age 65yr +, able to stand upright | Tanita; BF-350 | ASMMBIA= 7.1 + (−2.8 × gender) + (0.5 × BMI) + (0.1 × Max Grip Strength) + (−0.1 × FMBIA) gender: male = 0 female = 1 FMBIA fat mass from BIA (%) |
FNIH:* Male < 19.75 kg Female: < 16 kg EWGSOP2:* Male: < 7 kg/m2 Female: < 6 kg/m2 Two criteria led to same classification |
all | N=125 | 0.80 (0.66, 0.94) | 0.91 (0.85, 0.96) | 0.73 (0.58, 0.88) | 0.93 (0.88, 0.99) | 8.89 | 0.22 |
| Reiter 2019 21 | Geriatric inpatients 70yrs+, university hospital Salzburg Austria, able to walk a few metres and lie still for five minutes | AKERN BIA 101, Florence, Italy | 1. Kyle 2. Sergi 3. Scafoglieri 4. Rangel |
Baumgartner: Men: < 7.26 kg/m2 Women: < 5.5 kg/m2 FNIH (muscle weakness): Men: <0.789 Women: < 0.512 |
Baumgartner + Kyle | N=144 | 0.37 | 0.98 | 0.90 | 0.75 | 18.50 | 0.64 |
| Baumgartner + Sergi | N=144 | 0.55 | 0.97 | 0.90 | 0.81 | 18.33 | 0.46 | |||||
| Baumgartner + Scafoglieri | N=144 | 0.63 | 0.94 | 0.84 | 0.83 | 10.50 | 0.39 | |||||
| Baumgarnter + Rangel | N=144 | 0.61 | 0.90 | 0.75 | 0.82 | 6.10 | 0.43 | |||||
| FNIH + Kyle | N=144 | 0.28 | 0.98 | 0.85 | 0.78 | 14.00 | 0.73 | |||||
| FNIH + Sergi | N=144 | 0.38 | 0.98 | 0.88 | 0.80 | 19.00 | 0.63 | |||||
| FNIH + Scafoglieri | N=144 | 0.55 | 0.95 | 0.82 | 0.85 | 11.00 | 0.47 | |||||
| FNIH + Rangel | N=144 | 0.52 | 0.95 | 0.81 | 0.84 | 10.40 | 0.51 | |||||
| Steihaug 2016 22 | Inpatients 65yrs+, university hospital, admitted for surgical repair of hip fracture, Bergen, Norway | Single frequency tetrapolar BIA (RJL, III systems quantum R USA) and Body impedance analyzer (BIA 1010 ASE, Akern Srl, Italy) | 1.Kyle 2.Tengvall 3.Janssen 4.Sergi |
Baumgartner: Men: < 7.26 kg/m2 Women: < 5.67 kg/m2 |
Kyle Men | N=42 | 0.60 (0.41, 0.79) | 1.00 (1.00, 1.00) | ∞ | 0.40 | ||
| Kyle Women | N=116 | 0.65 (0.52, 0.78) | 0.95 (0.90, 1.00) | 13.9 | 0.36 | |||||||
| Tengval Men | N=42 | 0.56 (0.37, 0.75) | 1.00 (1.00, 1.00) | ∞ | 0.44 | |||||||
| Tengvall Women | N=116 | 0.62 (0.48, 0.75) | 0.94 (0.88, 1.00) | 9.8 | 0.40 | |||||||
| Janssen Men | N=42 | 0.32 (0.14, 0.50) | 1.00 (1.00, 1.00) | ∞ | 0.68 | |||||||
| Janssen Women | N=116 | 0.42 (0.29, 0.56) | 0.97 (0.93, 1.00) | 13.1 | 0.60 | |||||||
| Sergi Men | N=42 | 0.84 (0.70, 0.98) | 0.94 (0.83, 1.00) | 14.3 | 0.17 | |||||||
| Sergi Women | N=116 | 0.66 (0.52, 0.78) | 0.90 (0.83, 0.98) | 6.9 | 0.38 | |||||||
| Reiss 2016 23 | Geriatric inpatients 70yrs+, university hospital Salzburg Austria, able to walk a few metres and lie still for five minutes (subset of n=144 21 | BIA single-frequency device (AKERN Florence, Italy) | Janssen equation sex=1 for men and 0 for women Ht in cm R total body resistance | Janssen cutoffs: Men: < 8.5 kg/m2 Women: < 5.75 kg/m2 Chien cutoffs: Men: < 8.87 kg/m2 Women: < 6.42 kg/m2 |
Janssen cutoffs | N=60 | 0.77 (0.59, 0.79) | 0.71 (0.58, 0.85) | 0.54 (0.35, 0.73) | 0.88 (0.77, 0.99) | 2.66 | 0.32 |
| Chien cutoffs | N=60 | 0.55 (0.33, 0.79) | 0.90 (0.82, 0.99) | 0.71 (0.48, 0.95) | 0.83 (0.72, 0.94) | 5.50 | 0.50 |
The diagnostic properties of the BIA based equations for diagnosing sarcopenia are reported in Table 2. Four samples, using three equations17,18,23 demonstrated that using BIA had a specificity of less than 0.72, while 19 samples using eight equations19–23 demonstrated that BIA had a specificity greater than 0.89. Similarly, four samples using four equations18,20,22,23 found a sensitivity greater than 0.76 while 19 samples using eight equations17,19,21–23 found that BIA had a sensitivity less than 0.67. Collectively, the specificity of BIA across the 10 unique equations ranged from 0.57 – 1, while the sensitivity ranged from 0.275 – 0.84.
Discussion
This systematic review identified seven studies that assessed the utility of BIA, alone or in combination with other variables, as a method of diagnosing sarcopenia in older adults. While criteria and protocols varied greatly, these studies found inconsistent sensitivity and specificity for equations that used BIA as a means to diagnose sarcopenia. This indicates that a wide degree of heterogeneity exists in the methods between studies that use BIA as a measurement tool when evaluating older adults. High degrees of heterogeneity were observed in whether a fasting protocol was described, patient demographics, and predictive equations used. This review identified 10 unique predictive equations that have been used in adults over the age of 60. The large number of predictive equations that have been developed highlights the variability involved in BIA as a diagnostic tool for sarcopenia, as it is significantly impacted by demographic factors such as race, age, and sex. A recently published systematic review by Beaudart et al.24 identified similar issues with BIA predictive equations across all age groups, identifying the need for BIA equations to be validated in their intended population prior to clinical implementation. Similarly, Gonzalez et al.25 identified that using BIA as a diagnostic tool for sarcopenia requires clear and specific conditions in order to be applicable in an older population. As such, it is imperative to standardize protocols and equations to the population of focus in a research setting before clinical recommendations can be made.
BIA as a diagnostic tool for sarcopenia
A primary issue in the diagnosis of sarcopenia using BIA was the lack of standardization for how sarcopenia was defined, and the lean body mass cut-offs associated. Several studies used BIA predictive equations with different estimates for low muscle mass. While different cut-offs for sarcopenia have been created by different working groups, the studies in this review did not consistently follow the suggested cut-off points. These differences in the definition of low lean body mass may have contributed to the variability in the prevalence of sarcopenia diagnosed in these studies. Due to inconsistencies across measurement protocols and lean body mass cut-off values, results of studies using BIA to determine the prevalence of sarcopenia may not reflect prevalence rates as determined by another form of lean mass measurement.
The majority of the studies used DXA as a reference standard for BIA. While DXA potentially offers the best surrogate measure for skeletal muscle mass in large clinical trials, it is by definition not a true evaluation of lean muscle mass.26,27 Known limitations currently exist when using DXA as part of the diagnosis of sarcopenia, including inconsistencies relating to its ability as a predictor of outcomes such as functional decline.27 Therefore, DXA may serve as the best reference standard in the measurement of lean mass, but issues may arise when the creation of predictive equations using BIA are built with DXA as the reference standard itself.
From a pragmatic standpoint, lean body mass measurement devices such as BIA may offer a better means of detecting sarcopenia in a hospital setting instead of a clinical setting, as low lean body mass is associated with hospital stays and surgical outcomes.20,21,23 However, functional measures for sarcopenia such as hand grip strength and gait speed may offer a more feasible option for outcomes considered more prevalent in a clinical setting, such as fall risk.28
BIA was found to have high specificity and low sensitivity when used to diagnose sarcopenia. This demonstrates similar utility to the SARC-F questionnaire and therefore, may not offer additional benefit.29 In the clinical setting, avoiding false negatives should be given priority in order to avoid missing people with sarcopenia. Therefore, implementing the SARC-F combined with a calf circumference measurement (SARC-CalF) is recommended, as it demonstrates greater sensitivity than using the SARC-F alone.30 This means that BIA equations used in the diagnosis of sarcopenia may not add benefit to pre-existing, more affordable, and more accessible tools. Given BIA’s inconsistencies in diagnosing sarcopenia, it may act as more of a barrier than an aid for patients who would benefit from conservative interventions for sarcopenia, such as nutritional intervention and exercise prescription.
Feasibility
BIA offers a feasible option for a home and clinical setting due to the portability, ease of use and financial costs associated. However, based on the findings of this review, significant challenges in BIA’s diagnostic ability still exist due to both the variability in predictive equations and the BIA device used. Furthermore, if measurement protocols (time of measurement, patient in fasting or fed state) are not repeatable, the results and utility of BIA is likely to be inconsistent and skewed.26
Limitations
There were several methodological differences between studies that offer potential barriers to interpretation on an individual level. Some of this heterogeneity was due to protocols for measurement, such as the device used, equation used, electrode placement, fasting protocol, sex, and geography. Studies used different definitions of sarcopenia and muscle mass cut-offs, which changes the interpretation for sarcopenia diagnostic studies. Several devices also had proprietary predictive formulas which presented a limitation for reviewers to reproduce the data. Studies also tended to avoid re-test which minimized the ability to determine overall reliability of BIA.
The use of the QAREL checklist to determine the quality of included studies appeared to have some challenges. This was related to the irrelevance of some items included and determining that the majority of studies lacked assessor blinding when using measurement techniques. No study was deemed ineligible based on the inherent limitations of the critical appraisal tool for this subject.
Conclusion
BIA offers a portable, easy-to-use, and affordable means to assess lean body mass. However, due to the high heterogeneity in methodology between studies, BIA cannot be confidently recommended for use to diagnose sarcopenia. At the present time, if BIA is to be used on an individual level for diagnostic purposes, it should be used cautiously and with an equation that has been validated in a study population that best represents the patient it is being used for. For clinicians seeking to screen patients for sarcopenia, more clinically feasible functional tests and validated screening questionnaires should be considered.
Future research should aim to standardize, explicitly state, and reproduce methodology related to factors such as, sarcopenia cut-off definition, fasting or fed state, type of device, equation used, and patient demographics. As the number of older adults continues to rise, it becomes paramount to develop clinically useful and reliable means of measuring muscle mass and strength to diagnose sarcopenia.
Appendix 1. Electronic search strategy and results
Database(s): Ovid MEDLINE: Epub Ahead of Print, In-Process & Other Non-Indexed
Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® 1946-Present
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | Sarcopenia/ | 4060 |
| 2 | Muscular Atrophy/ | 10822 |
| 3 | Body Composition/ | 41750 |
| 4 | body mass index/ | 124823 |
| 5 | Muscle, Skeletal/ | 140138 |
| 6 | sarcopen*.ti,ab. | 7499 |
| 7 | ((muscle* or muscular) adj3 atroph*).ti,ab. | 18293 |
| 8 | ((muscle* or muscular*) adj3 degener*).ti,ab. | 3714 |
| 9 | ((muscle* or muscular*) adj3 wast*).ti,ab. | 5579 |
| 10 | (body adj3 composition*).ti,ab. | 36968 |
| 11 | (body adj2 mass).ti,ab. | 214319 |
| 12 | (muscle adj2 mass).ti,ab. | 18219 |
| 13 | (lean adj2 mass).ti,ab. | 13194 |
| 14 | (fat-free adj2 mass).ti,ab. | 7733 |
| 15 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 | 466592 |
| 16 | Aged/ | 3040981 |
| 17 | Aging/ | 226638 |
| 18 | “Aged, 80 and over”/ | 899385 |
| 19 | centenarian*.ti,ab. | 1966 |
| 20 | (elderly or elders or elder).ti,ab. | 252187 |
| 21 | geriatric*.ti,ab. | 46483 |
| 22 | (nonagenarian* or octogenarian*).ti,ab. | 4267 |
| 23 | old age home*.ti,ab. | 294 |
| (old adj2 (women or men or adult* or people* or population* or person or | ||
| 24 | persons)).ti,ab. | 23524 |
| (older adj2 (women or men or adult* or people* or population* or person or | ||
| 25 | persons)).ti,ab. | 144408 |
| 26 | (old-age* or older patient*).ti,ab. | 66501 |
| 27 | (post-menopaus* or postmenopaus*).ti,ab. | 60664 |
| 28 | 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 | 3398937 |
| 29 | Electric Impedance/ | 16888 |
| 30 | Electric Conductivity/ | 32445 |
| 31 | (electric* adj2 imped*).ti,ab. | 4752 |
| 32 | (bioelectric* adj2 imped*).ti,ab. | 5809 |
| 33 | 29 or 30 or 31 or 32 | 53698 |
| 34 | exp “Sensitivity and Specificity”/ | 578257 |
| 35 | exp “reproducibility of results”/ | 396902 |
| 36 | “Predictive Value of Tests”/ | 200383 |
| 37 | likelihood functions/ | 21864 |
| 38 | False Positive Reactions/ | 27581 |
| 39 | false negative reactions/ | 17639 |
| 40 | roc curve/ | 56971 |
| 41 | Odds Ratio/ | 89678 |
| 42 | exp Statistics as Topic/ | 2698000 |
| 43 | statistics, nonparametric/ | 72479 |
| 44 | exp Regression Analysis/ | 420312 |
| 45 | (accura* or correlat* or co-relat* or corelat* or cross-valid*).ti,ab. | 2532540 |
| 46 | (false* adj2 negative*).ti,ab. | 33666 |
| 47 | (false* adj2 positiv*).ti,ab. | 59721 |
| 48 | (internal* adj2 consistenc*).ti,ab. | 29530 |
| (intra-rater* or inter-rater* or interrater* or intrarater* or rater* or intra-examiner* | ||
| 49 | or inter-examiner* or intraexaminer* or interexaminer*).ti,ab. | 30013 |
| 50 | kappa coefficien*.ti,ab. | 5904 |
| 51 | (likelihood* adj2 (function* or ratio*)).ti,ab. | 16756 |
| 52 | (linear adj2 (model* or regression*)).ti,ab. | 152503 |
| 53 | (mean adj2 differen*).ti,ab. | 75448 |
| 54 | (multivariate adj2 analy*).ti,ab. | 208844 |
| 55 | (odds adj2 ratio*).ti,ab. | 268667 |
| 56 | (predictive* adj2 value*).ti,ab. | 107752 |
| 57 | (r-coefficient* or “r coefficent”).ti,ab. | 247 |
| 58 | reference value.ti,ab. | 3583 |
| 59 | reliab*.ti,ab. | 475255 |
| 60 | reproducibility.ti,ab. | 75212 |
| 61 | responsiveness*.ti,ab. | 100533 |
| 62 | (roc curve* or received operating characteristic*).ti,ab. | 32679 |
| 63 | sensitivity*.ti,ab. | 786967 |
| 64 | specificity*.ti,ab. | 456410 |
| 65 | test-retest*.ti,ab. | 25702 |
| 66 | (pearson* or spearman*).ti,ab. | 82763 |
| 67 | (utility* adj2 test*).ti,ab. | |
| 68 | valid*.ti,ab. | 721043 |
| 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or | ||
| 69 | 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or | 6457990 |
| 62 or 63 or 64 or 65 or 66 or 67 or 68 | ||
| 70 | 15 and 28 and 33 and 69 | 1645 |
| (comment or editorial or letter or clinical conference or review or guideline or | ||
| 71 | 6221554 practice guideline or case reports).pt. | |
| 72 | 70 not 71 | 1596 |
Footnotes
The authors have no disclaimers, competing interests, or sources of support or funding to report in the preparation of this manuscript.
References
- 1.Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50(5):1231–1233. [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anker SD, Morley JE, vonHaehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–514. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH Sarcopenia Project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020;68(7):1410–1418. doi: 10.1111/jgs.16372. [DOI] [PubMed] [Google Scholar]
- 7.Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Mijnarends DM, Meijers JMM, Halfens RJG, ter Borg S, Luiking YC, Verlaan S, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc. 2013;14(3):170–178. doi: 10.1016/j.jamda.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8(5):702–712. doi: 10.1002/jcsm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyle U. Bioelectrical impedance analysis – part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res. 2017;29(4):591–597. doi: 10.1007/s40520-016-0622-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Choi SH, Lim S, Kim KW, Lim JY, Cho NH, et al. Assessment of appendicular skeletal muscle mass by bioimpedance in older community-dwelling Korean adults. Arch Gerontol Geriatr. 2014;58(3):303–307. doi: 10.1016/j.archger.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida D, Shimada H, Park H, Anan Y, Ito T, Harada A, et al. Development of an equation for estimating appendicular skeletal muscle mass in Japanese older adults using bioelectrical impedance analysis. Geriatr Gerontol Int. 2014;14(4):851–857. doi: 10.1111/ggi.12177. [DOI] [PubMed] [Google Scholar]
- 14.Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–952. doi: 10.1016/j.jclinepi.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16(1):21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas NP, Macaskill P, Irwig L, Bogduk N. The development of a quality appraisal tool for studies of diagnostic reliability (QAREL) J Clin Epidemiol. 2010;63(8):854–861. doi: 10.1016/j.jclinepi.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Kimura Y, Ishiyama D, Nishio N, Abe Y, Kakehi T, et al. Differential characteristics of skeletal muscle in community-dwelling older adults. J Am Med Dir Assoc. 2017;18(9):807e9–807.e16. doi: 10.1016/j.jamda.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Laksmi PW, Sukma FA, Setyohadi B, Nugroho P, Ariane A, Tirtarahardja G. The need for a new cut-off value to increase diagnostic performance of bioelectrical impedance analysis compared with dual-energy x-ray absorptiometry to measure muscle mass in Indonesian elderly. Acta Med Indones. 2019;51(2):7. [PubMed] [Google Scholar]
- 19.Fang W-H, Yang J-R, Lin C-Y, Hsiao P-J, Tu M-Y, Chen C-F, et al. Accuracy augmentation of body composition measurement by bioelectrical impedance analyzer in elderly population. Medicine (Baltimore) 2020;99(7):e19103. doi: 10.1097/MD.0000000000019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deer RR, Akhverdiyeva L, Kuo Y, Volpi E. Developing a screening tool for sarcopenia in hospitalized geriatric patients: estimation of appendicular skeletal muscle mass using bioelectrical impedance. Clin Nutr Edinb Scotl. 2020;39(7):2233–2237. doi: 10.1016/j.clnu.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter R, Iglseder B, Treschnitzer W, Alzner R, Mayr-Pirker B, Kreutzer M, et al. Quantifying appendicular muscle mass in geriatric inpatients: performance of different single frequency BIA equations in comparison to dual X-ray absorptiometry. Arch Gerontol Geriatr. 2019;80:98–103. doi: 10.1016/j.archger.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Steihaug OM, Gjesdal CG, Bogen B, Ranhoff AH. Identifying low muscle mass in patients with hip fracture: validation of bioelectrical impedance analysis and anthropometry compared to dual energy x-ray absorptiometry. J Nutr Health Aging. 2016;20(7):685–690. doi: 10.1007/s12603-016-0686-1. [DOI] [PubMed] [Google Scholar]
- 23.Reiss J, Iglseder B, Kreutzer M, Weilbuchner I, Treschnitzer W, Kässmann H, et al. Case finding for sarcopenia in geriatric inpatients: performance of bioimpedance analysis in comparison to dual x-ray absorptiometry. BMC Geriatr. 2016;16(1):52. doi: 10.1186/s12877-016-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaudart C, Bruyère O, Geerinck A, Hajaoui M, Scafoglieri A, Perkisas S, et al. Equation models developed with bioelectric impedance analysis tools to assess muscle mass: a systematic review. Clin Nutr ESPEN. 2020;35:47–62. doi: 10.1016/j.clnesp.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle. 2017;8(2):187–189. doi: 10.1002/jcsm.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9(2):269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10(1):14–21. doi: 10.1002/jcsm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68(7):1429–1437. doi: 10.1111/jgs.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahat G, Yilmaz O, Kiliç C, Oren MM, Karan MA. Performance of sarc-f in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging. 2018;22(8):898–903. doi: 10.1007/s12603-018-1067-8. [DOI] [PubMed] [Google Scholar]
- 30.Mo Y, Dong X, Wang X. Screening accuracy of sarc-f combined with calf circumference for sarcopenia in older adults: a diagnostic meta-analysis. J Am Med Dir Assoc. 2020;21(2):288–289. doi: 10.1016/j.jamda.2019.09.002. [DOI] [PubMed] [Google Scholar]