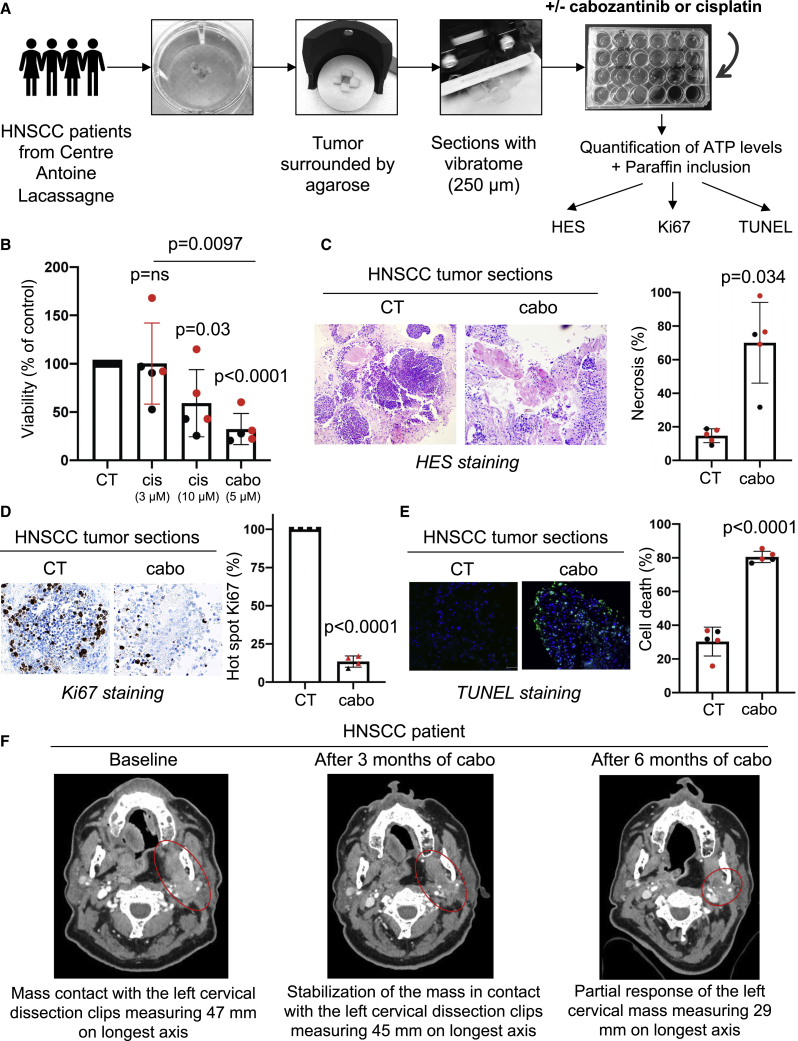
Figure 7.
Cabozantinib is efficient on three-dimensional (3D) viable sections of surgically resected HNSCC
Samples of HNSCC obtained from patients (N = 5) following surgery were analyzed by a pathologist (HNSCC samples, Table S2). The tumor samples were surrounded by agarose, and viable 3D tumor sections (250 μm) were obtained with a vibratome. Viable 3D tumor sections were cultured in a specific medium and treated for 96 h with cabozantinib (5 μM) or cisplatin (3 and 10 μM). The results of the sections of tumors from diagnosed patients are shown in black and from patients who relapsed after cisplatin and radiotherapy are shown in red in the different panels.
(A) The experimental protocol is shown.
(B) The concentration of ATP provides a readout of the tumor section viability.
(C–E) Viable 3D tumor sections were paraffin embedded and stained with HES to quantify the areas of necrosis (C), with Ki67 to quantify cell proliferation (D), and with TUNEL to quantify apoptotic cells (E). Representative images are shown. Statistics were performed using the ANOVA test, and p values are indicated.
(F) An HNSCC patient treated with cabozantinib. Mr. A had an HNSCC and was treated in fifth line with 60 mg/day cabozantinib from January to September 2021 at the Centre Antoine Lacassagne. The tumor burden was stable form December 2020 to March 2021 followed by a partial response from March to September 2021. Computed tomography (CT) scans of the tumor mass enhanced with contrasting product are shown (baseline, after 3 months of cabozantinib, and after 6 months of cabozantinib).