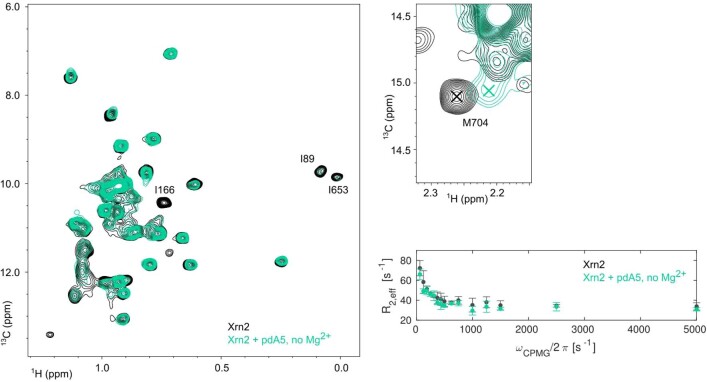
Extended Data Fig. 8. In the absence of Mg2+ ions pdA5 interacts with the RNA binding pocket, but does not induce the active conformation in Xrn2.
Overlay of the 1H-13C HMQC methyl-TROSY spectra of the apo state (black) and after addition of pdA5 (green), recorded at 18.8 T and 313 K. CSPs of M704 (right panel) clearly report on the interaction of pdA5 with the RNA-binding pocket. Spectra are recorded in the absence of Mg2+. The absence of CSPs around the active site (as observed in the presence of Mg2+; Fig. 4G) indicates that the stable active conformation is not formed when Mg2+ is not present. Binding of pdA5 in the absence of Mg2+ does not change the dynamics of the α1-helix (opposed to what was observed in the presence of Mg2+; Fig. 4H), as shown by the overlay of 19F CPMG data in the absence (black) and presence (green) of pdA5. Data points are shown as mean ± s.d., as derived from 3 duplicate NMR measurements.