Fig. 1. Antibody responses against SARS-CoV-2 across severe versus critical clinical course of disease.
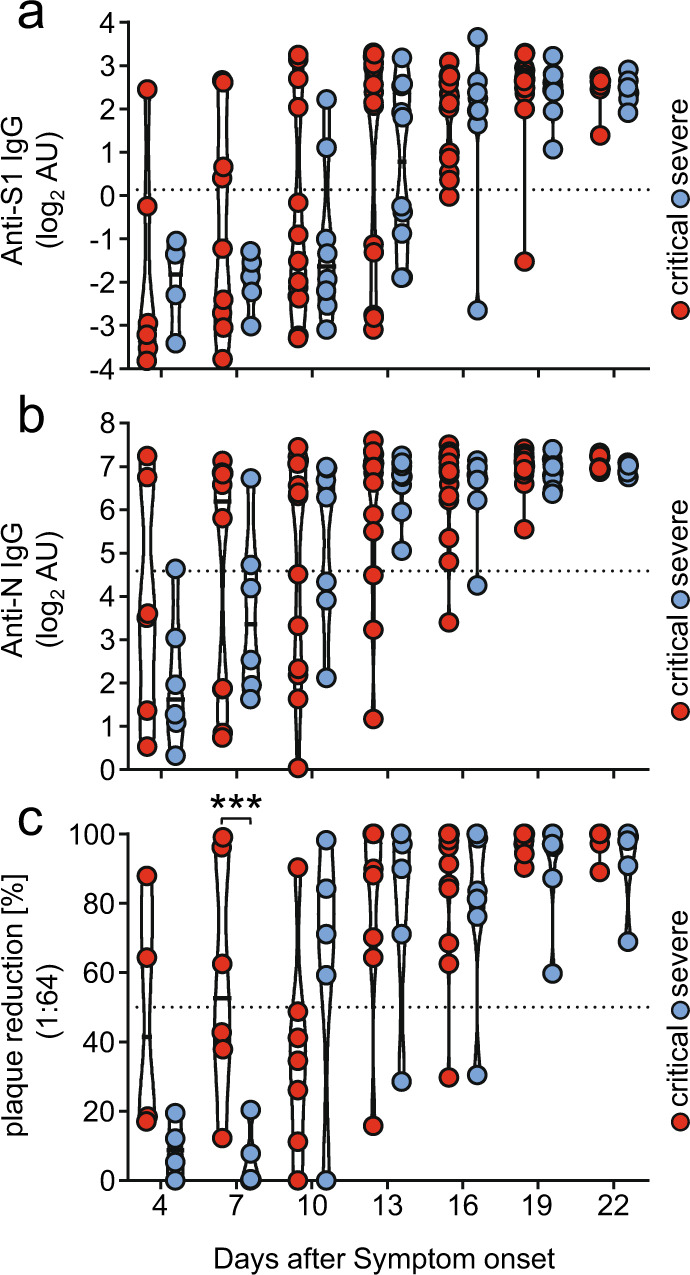
IgG antibody levels were analyzed in longitudinal serum samples from hospitalized SARS-CoV-2 infected individuals. Patients were categorized as critically diseased when in need of invasive mechanical ventilation (n = 27; red symbols) compared to severely diseased patients who did not require invasive ventilation (n = 14; blue symbols). Each dot represents the mean value obtained by the analysis of all samples, which were available at the indicated time points (+/− 1 day) following symptom onset. a IgG response against SARS-CoV-2 S1 –protein and b SARS-CoV-2 N-protein as determined by commercial ELISA assays. Dotted lines represent cut-off values for commercial S1- and N- specific ELISA assays. Each dot represents the mean value obtained by the analysis of all samples, which were available at the indicated time points (+/− 1 day) following symptom onset. c Serum neutralization capacity against SARS-CoV-2 measured by a plaque reduction assay. Sera were considered neutralizing at 50% plaque reduction (dotted line) at a 1:64 dilution. Solid black lines indicate the median. Significant differences were tested using a linear mixed effects model (one-sided, no adjustments) (***p < 0.001). Source data are provided as a Source Data file.
