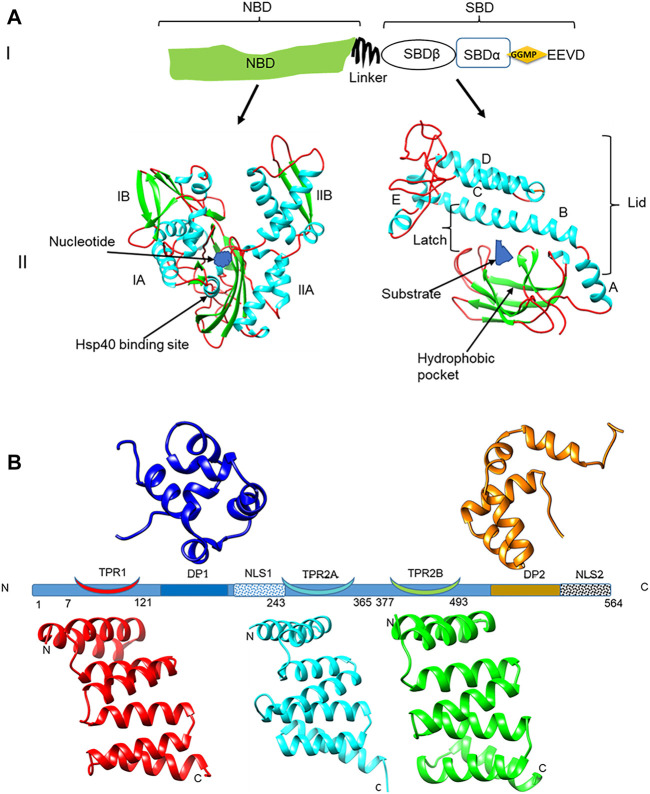
FIGURE 1.
Domain representation of PfHsp70-1 and PfHop (A) (panel I): representation of the linear structural organization of PfHsp70-1 showing the NBD, a highly charged linker, and the-SBD. The C-terminal EEVD motif is illustrated. Panel II: Three-dimensional model of PfHsp70-1 domains. The N-terminal NBD which is subdivided into lobes IA, IB, IIA, and IIB, respectively, is shown. Also illustrated are the bound nucleotide (blue) and the Hsp40 co-chaperone binding site, respectively. The C-terminal SBD of the protein is shown on the right hand side. The hydrophobic pocket located in the SBD and a bound peptide substrate are also shown. The alpha-helical lid which is made up of helices A, B, C, D, and E is also depicted as adapted from Shonhai (2007). (B) Depiction of the structural organization of PfHop showing the relative positions of the three tetracopeptide repeat (TPR) regions, the two dipeptide domains (DP), and the two nuclear localization signals (NLS) of the protein. Ribbon representations of the three-dimensional models of TPR1, TPR2A, TPR2B, DP1, and DP2 domains are also shown. The models were generated using PHYRE2 (http://www.sbg.bio.ic.ac.uk/phyre2; Kelley et al., 2015) and rendered using CHIMERA version 1.15rc (Pettersen et al., 2004).