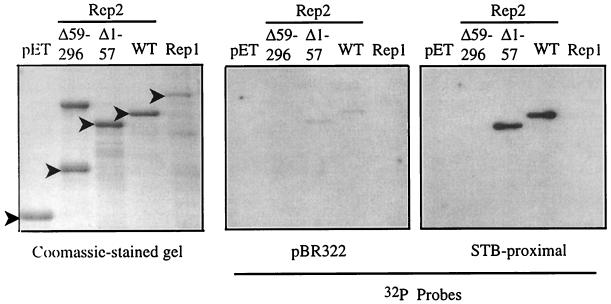
FIG. 4.
Southwestern assay shows Rep2 DNA-binding activity. pET-Rep fusion proteins were purified by affinity chromatography and separated by SDS-PAGE. Triplicate gels were either stained with Coomassie blue or Western blotted and incubated with a radiolabeled DNA (either a 375-bp EcoRI-BamHI fragment from the E. coli plasmid pBR322 or a 312-bp EcoRI-HindIII fragment containing the 2μm STB-proximal locus), and the signal was detected by autoradiography. The positions of the pET-Rep fusion proteins are indicated by filled arrowheads. (A higher-molecular-weight protein that copurifies with the pET-Rep2Δ59–296 fusion protein on Talon resin is observed as a slower-migrating species on the Coomassie blue-stained gel.) WT, wild type.