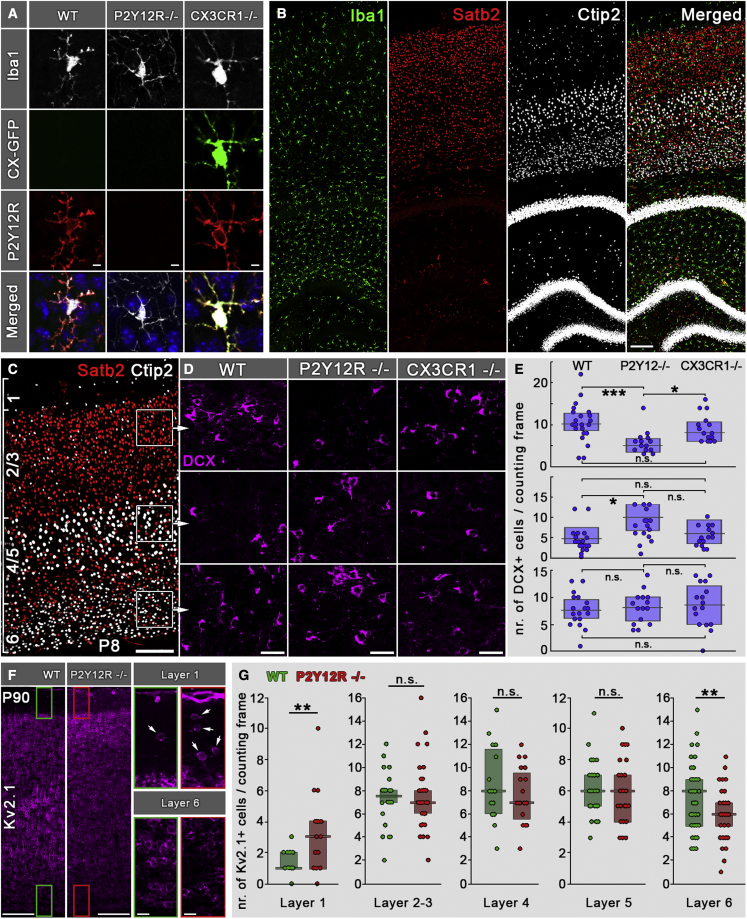
Figure 5.
Microglial P2Y12Rs are necessary for formation of proper cortical cytoarchitecture
(A) CLSM images showing triple immunofluorescence staining for Iba1, GFP, and P2Y12Rs on the different genotypes used in this experiment. Merged images also show cell nuclei labeled with DAPI.
(B) CLSM image showing triple immunofluorescence staining for Iba1, Ctip2, and Satb2 in P8 mice.
(C–E) Ctip2 and Satb2 immunofluorescence staining was used to delineate cortical layers, and the density of DCX+ cells was assessed in layers 6, 4/5, and 2/3.
(D and E) In layer 6, there was no difference in DCX+ cell density between WT, P2Y12R−/−, and CX3CR1−/− mice (n = 9 mice). However, in layers 4/5, we observed a significant (200% of WT) increase in DCX+ cell density in P2Y12R−/− mice compared with WT mice, which did not differ from CX3CR1−/− mice (n = 9 mice). On the contrary, in layers 2/3, the density of DCX+ cells was significantly lower (50% of WT) in P2Y12R−/− mice compared with WT or CX3CR1−/− mice, which did not differ from each other (n = 9 mice). Median values and interquartile range are marked by boxes and whiskers. Kruskal-Wallis test followed by Tukey’s comparison; ∗p < 0.05, ∗∗∗p < 0.001.
(F) Kv2.1 staining in adult mouse neocortex in WT and P2Y12R KO animals; insets show enlarged areas from layers 1 and 6.
(G) Lack of P2Y12Rs causes robustly elevated neuronal density in layer 1 (300% over WT) and leads to a significant decrease of neuronal density in layer 6 (75% of WT, n = 6 mice). Median values and interquartile ranges are marked by boxes and whiskers. Mann-Whitney U test; ∗∗p < 0.01.
Scale bars represent 5 μm in (A), 100 μm in (B) and (C), 20 μm in (D), 150 μm in (F), and 20 μm in insets.