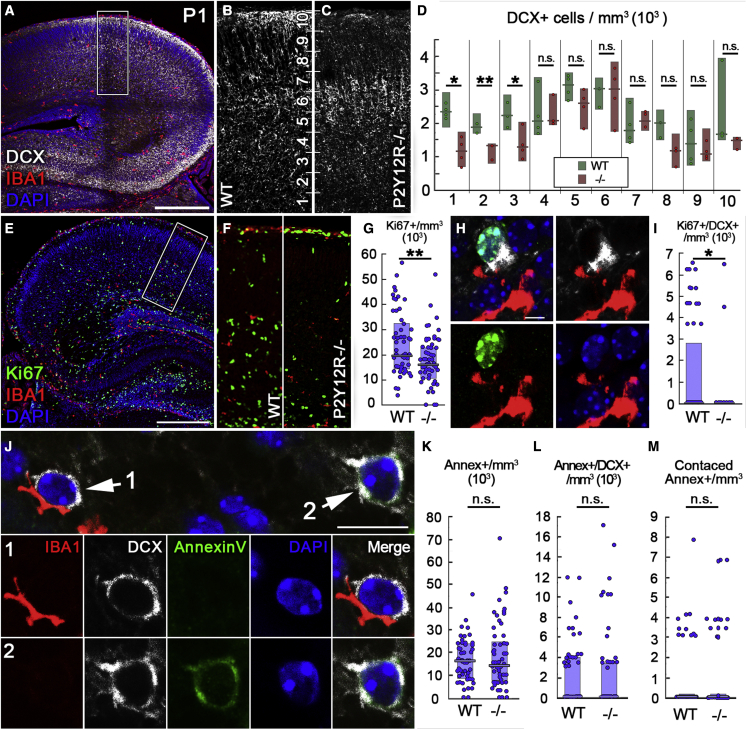
Figure 6.
Microglial P2Y12R signaling is necessary for proper neuronal proliferation in the developing neocortex
(A–C) Maximum intensity projection of a 20-μm-thick volume from a CLSM stack from an P1 mouse (A). DCX+ neurons are shown in white and IBA1+ microglia in red, and nuclei are visualized by DAPI (blue). The area within the white box is enlarged in (B) and (C). The cortex is divided into 10 zones, numbered from the border of the SVZ to the pial surface.
(D) Lack of P2Y12Rs causes decreased density of DCX+ cells in the lower zones (to 51% of WT in zone 1, 72% of WT in zone 2, and to 58% of WT in zone 3; n = 6 mice). Mann-Whitney U test; ∗p < 0.05, ∗∗p < 0.01.
(E) CLSM images showing triple immunofluorescence staining for Ki67 (green), IBA1 (red), and DAPI (blue).
(F) The genotypes and the areas of measurements are the same as above.
(G) Lack of P2Y12Rs causes decreased density of Ki67+ cells to 82% of WT; n = 6 mice. Mann-Whitney U test, ∗∗p < 0.01.
(H) A population of DCX+ cells is also positive for Ki67.
(I) Lack of P2Y12Rs causes a significant decrease of the density of Ki67/DCX double-positive cells (to 0% of WT, n = 6 mice). Mann-Whitney U test, ∗p < 0.05.
(J) CLSM image showing immunofluorescence staining for Iba1 (red), DCX (white), Annexin V (green), and DAPI (blue). The images below show microglia contacting a DCX+ cell (1) and an Annexin V/DCX double-positive cell without microglial contact (2).
(K) Density of Annexin V+ cells in WT and P2Y12R−/− animals (n = 6 mice).
(L) Density of Annexin V/DCX double-positive cells in WT and P2Y12R−/− animals (n = 6 mice).
(M) Density of Annexin V+ cells contacted by microglia in WT and P2Y12R−/− animals (n = 6 mice). Mann-Whitney U test. Median values and interquartile ranges are marked by boxes and whiskers.
Scale bars represent 300 μm in (A) and (E), 5 μm in (H), and 10 μm in (J). Insets are 200 μm wide in (B) and (C) and 150 μm wide in (F). See also Figures S4 and S5.