FIGURE 3.
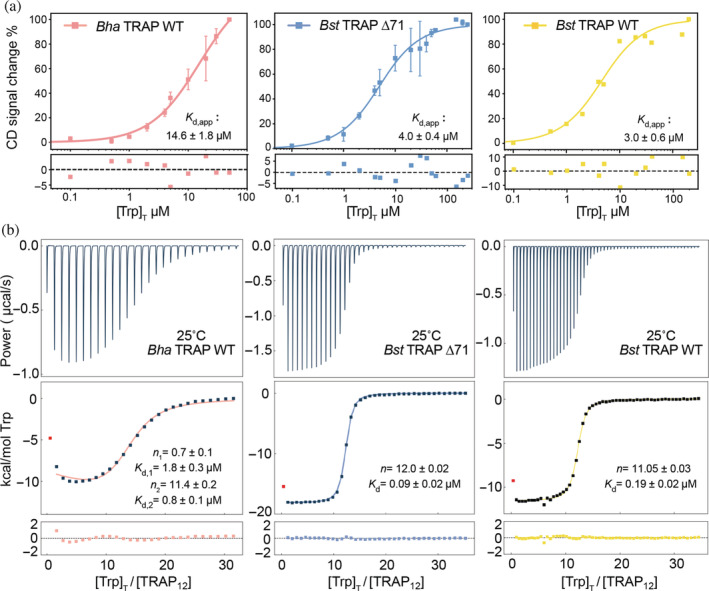
Bulk binding and phenomenological modeling provide limited information on cooperative ligand‐binding to dodecameric Bha TRAP and Bst TRAP Δ71 (left, middle), or undecameric wild‐type Bst TRAP (right). (a) The circular dichroism signal at 228 nm in 50 mM sodium phosphate buffer (pH = 8) was measured as a function of added Trp, and the data fit with the quadratic binding equation (Equation (1); Table S1). CD data were acquired by using 2 μM TRAP with increasing Trp concentration from 0 to 300 μM. The fitted parameters from CD suggest all three proteins exhibit similar μM‐binding affinity and homotropic cooperativity is not evident. (b) ITC experiments titrating Trp into TRAP at 25°C in buffer A (see Methods) were fit with quadratic independent site models (Table S2). Bst TRAP Δ71 (middle) and Bst TRAP (right) can be well fitted with one‐site binding model, in which 12mer Bst TRAP Δ71 (K d = 0.09 ± 0.02 μM) has two‐times higher binding affinity than 11mer Bst TRAP (K d = 0.19 ± 0.02 μM). The data for Bha TRAP (left) are better fit to a two‐sites binding model yielding parameters in which the first Trp binds with K d of 1.8 ± 0.3 μM while the remaining 11 bind with a K d of 0.8 ± 0.1 μM. CD, circular dichroism; TRAP, trp RNA‐binding attenuation protein
