FIGURE 4.
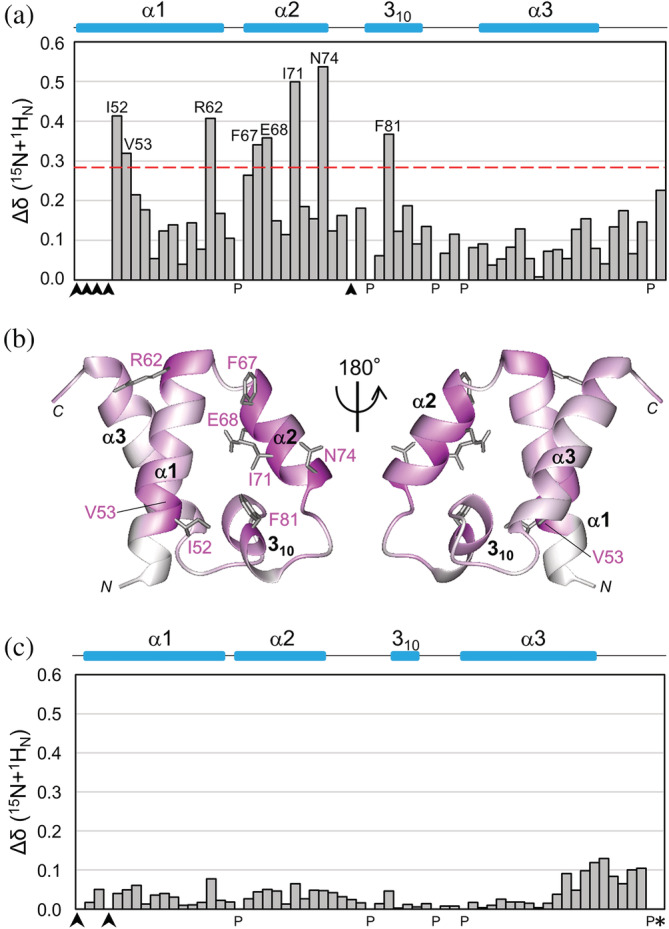
NMR chemical shift perturbations of labeled SURP1 upon binding of non‐labeled S1BRp, and comparison of chemical shift values between complex structure SURP1 and SURP1 of the chimera. (a) Quantification of the chemical shift perturbation values of labeled SURP1 upon S1BRp binding. Perturbation values were obtained from [1H,15N]‐HSQC spectra in the absence and presence of S1BRp (Figure S4a). The absolute values of the chemical shift change Δδ(15 N + 1 H N) are shown. The value for each residue was calculated as follows: Δδ(15 N + 1 H N) = [(δ 15N/6.5)2 + δ 1H 2]1/2. Perturbation values greater than the average (0.16 ppm) plus the standard deviation (0.12 ppm) were defined as significant perturbations (i.e., the significance level of 0.28 ppm is indicated by a red dotted line). Residues with resonances that were not assigned are indicated by arrowheads, and proline is indicated by P. Only residues with significant chemical shift changes are shown. (b) Mapping of residues with chemical shift changes on the ribbon representation of individual SURP1 in its free form [PDB ID 2DT7]. Residues are colored based on the magnitude of the chemical shift change upon S1BRp binding, ranging from white (not assigned or measured) to magenta (largest chemical shift change). Only the side chains of the residues with significant chemical shift changes are represented in gray. (c) Differences of the chemical shift values between the S1BRp‐bound form of SURP1 and the chimeric form of SURP1 in the chimera. The values were obtained from [1H,15N]‐HSQC spectrum of labeled SURP1 in the presence of S1BRp and from that of the labeled chimera (Figure S4b). The absolute values of the chemical shift difference between the two forms Δδ(15 N + 1 H N) are shown. The value for each residue was calculated, as described above. The average and standard deviation among residues in SURP1 are 0.04 ppm and 0.03 ppm, respectively, except for Gly110, which is the last residue of SURP1 (indicated by an asterisk). The absolute value of the chemical shift change of Gly110 is 6.19 ppm; the corresponding bar is not indicated in this graph. Because, in the chimera, Gly110 is directly connected with the linker, the chemical shift value cannot be simply compared with that of the last residue, Gly, of individual SURP1.
