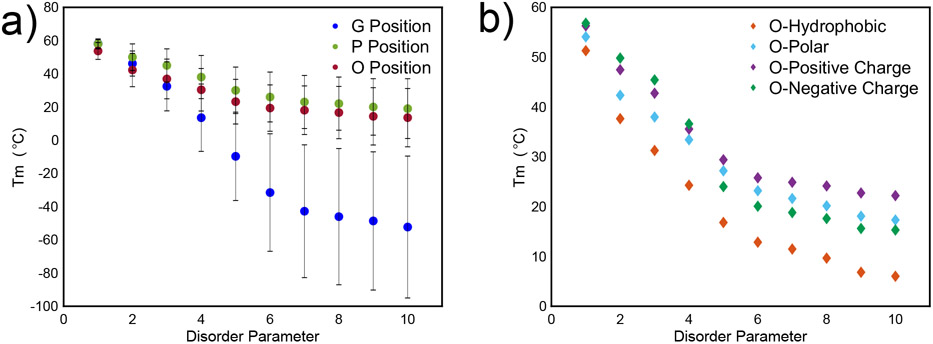
Figure 5. Characterization of effect of disorder on Tm, as predicted by the model.
A) Tm values of disorder arranged by G, P, or O position confirm that increasing mutations along the chain decreases thermal stability of the triple helix. Error bars indicate standard deviation of all amino acids that were mutated. b) Tm values of disorder in the O position demonstrates that initial mutations to polar, positive charged, and negative charged amino acids confer the same degree of stability in the molecule. However, upon increasing mutations, polar amino acids are the least destabilizing to the triple helix, suggesting that they should be used for bacterial expression of collagen where expression of O is not possible.