Abstract
Recurrent miscarriage (RM) is a complicated disease in reproductive medicine that impacts many families. Currently, the etiology of RM is thought to include chromosome abnormalities, reproductive tract malformations, autoimmune dysfunction, infection, and environmental factors. However, the underlying mechanisms of RM remain unknown. At present, research on long non-coding RNAs (lncRNAs) is rapidly emerging and becoming a hot research topic in epigenetic studies. Recent studies revealed that lncRNAs are strongly linked to RM and play a crucial role in epigenetic, cell cycle, cell differentiation regulation, and other life activities. This article mainly reviews the difference in lncRNA expression in patients with RM and regulation of susceptibility, endometrial receptivity, and the maternal-fetal interface. Meanwhile, the correlation between lncRNAs and RM is expounded, which provides new insights for the early diagnosis and treatment of RM.
Keywords: lncRNA, Recurrent miscarriage, Susceptibility, Endometrial receptivity, Maternal-foetal interface
Introduction
Recurrent miscarriage (RM) refers to the pregnancy loss before 20 weeks of gestation or a fetal weight less than 500 g for two or more consecutive pregnancies [1]. Most RM occurs when they are clinically diagnosed as occurring in the pre-embryonic or embryonic stage [2]. Early pregnancy loss occurs in approximately 11% of pregnant women at 8–12 gestational weeks [3], and the incidence of RM is about 2% among all pregnancy outcomes [4].
RM is a significant clinical problem in reproductive health and affects family well-being, but unfortunately, more than half of RM patients are unable to identify the etiological factors [5]. The leading causes of RM are maternal chromosomal abnormalities, genital tract abnormalities, immune dysfunction, endocrine disorders, the presence of genital tract infection, and cervical insufficiency [6]. Although chromosomal abnormalities are currently considered the leading cause of RM, there are still most cases where the cause is not yet known.
RNA sequencing, gene expression profiling by microarray and transcriptome analysis increasingly indicate the role of non-coding RNAs (ncRNAs) as critical regulators of gene expression and signal transduction [7]. And yet, only 2% of the human transcribed genome is protein-coding genes. Long non-coding RNAs (lncRNAs) are a specific type of ncRNAs, which play significant regulatory roles in health and disease [8–11]. LncRNAs transcripts are to participate in epigenetic remodeling, subcellular localization and transcriptional regulation. Aberrantly regulated lncRNAs are implicated in cancer through downregulation or upregulation of the specific lncRNAs associated with the adjacent normal tissue [12]. Consequently, lncRNAs act similarly to tumor proto-oncogenes or suppressor genes.
Studies have confirmed that long non-coding RNAs are involved in RM and regulate embryonic development, endometrial receptivity, and embryo-maternal interactions by regulating gene expression. Consequently, it is imperative for RM to explore the underlying mechanisms and seek new treatments in order to reduce the harm to families.
Overview of lncRNAs
LncRNAs are nucleic acid sequences that are lengths greater than 200 nt and do not encode proteins [13]. LncRNAs are mainly transcribed by RNA polymerase II and have an mRNA-like structure, typically with a 7mC cap at the 5′ end and a polyA tail at the 3′ end [14]. They account for approximately 27% of human-annotated genes and have a variety of mechanisms, which distinguish them from other small non-coding RNAs, such as miRNAs, piRNAs, siRNAs, and several others [15].
Accumulating evidence has indicated that lncRNA plays a critical role in biological processes. According to their genome location and background, lncRNAs can be divided into intronic lncRNAs, sense lncRNAs, antisense lncRNAs, intergenic lncRNAs, and bidirectional lncRNA [16]. The intronic lncRNA is derived from the intronic region of genes encoding proteins, originating from the antisense and sense regions of the intronic region, such as SPRY4-ITI and CHRF [17, 18]. The sense and antisense lncRNA are transcribed from the sense and antisense strands of the genome encoding protein genes, like ANRIL and COLDAIR [18, 19]. Intergenic lncRNA is known as large intervening non-coding RNA, transcribed from the intergenic region of genes encoding proteins, such as MALAT1, MIAT, and H19, etc. [17, 18]. Bidirectional lncRNA is originated from different directions of protein-coding genes, including HCCL5 and LEENE [19]. Although lncRNAs do not encode proteins, they play crucial roles in various biological processes. Firstly, lncRNAs exist in almost all living things, suggesting that they are primary components of organisms. LncRNAs account for a high proportion of RNAs in complex organisms, indicating that they serve a significant role in increasing complexity of eukaryotes [20]. Secondly, lncRNAs have high levels of tissue-specific expression, which is specifically manifested in the fact that the lncRNAs expression levels in diverse tissues are different, and distinct expression patterns are present in other parts of the same tissue [21]. Furthermore, lncRNAs have significant temporal and spatial specificity, and the expression of the same lncRNA varies significantly in different developmental stages of the same tissue or organ [22].
In early studies, due to the low level of transcription of lncRNAs, they were considered the “noise” of transcription [23]. However, with the improvement of experimental technology, the function of lncRNAs was found to be closely associated with their location. The use of FISH and other tools to determine the subcellular location of lncRNAs is conducive to studying their mechanism of action [24]. LncRNAs located in the nucleus can participate in gene regulation processes, including promoter-specific inhibition, transcription activation, and epigenetic regulation [25]. LncRNA regulates gene expression by directly binding RNA polymerase and transcription factors or by interfering with the binding of promoters and polymerase [26]. Moreover, lncRNAs regulate the chromatin structure through different functional steps, including histone modification, DNA methylation, and chromatin remodeling [27]. Additionally, lncRNAs located in the cytoplasm participate in post-transcriptional gene regulation, including the regulation of mRNA stability, miRNA translation, and signal transduction pathways [28]. LncRNAs facilitate the post-transcriptional processing of mRNA by recognizing complementary sequences in mRNA, such as splicing, transport from the nucleus to the cytoplasm, editing, etc., to obtain a mature form [29]. Meanwhile, lncRNAs act as a sponge, also called competitive endogenous RNAs (ceRNAs), containing sequences complementary to miRNA sequences, thereby isolating miRNA sequences and preventing them from binding to the target [29]. Moreover, lncRNAs may promote or inhibit translation by interacting with initiation factors, ribosomes, or ribosomal RNA [16]. The above findings suggest that lncRNAs can alter the stability of cells and tissues through these functions, which in turn can cause a variety of diseases. There is accumulating evidence for the critical role of lncRNAs in the tumourigenesis and progression of Hepatocellular carcinoma (HCC). A variety of HCC-associated lncRNAs have been proven to be aberrantly expressed and involved in cancer phenotypes (for example, sustained proliferation, evasion of apoptosis, accelerated angiogenesis and acquisition of invasive capacity) through binding to DNA, RNA or proteins or encoding small peptides [30]. Additionally, PCGEM1 introduces the Pygopus family PHD finger 2 into the enhancer-promoter region of the AR gene, modulates AR-induced gene expression, is overexpressed in prostate cancer, and promotes cell proliferation [31]. In breast cancer, lncRNA ANRIL induces gene silencing at the INK4b-ARF-INK4a locus by interacting with CBX7 (PRC1 component) and SUZ12 (PRC2 component) and regulates its adjacent tumor suppressor CDKN2A/B through epigenetic mechanisms, thereby controlling cell proliferation and senility [32]. The lncRNA CCAT1 acts as a competitive endogenous RNA (ceRNA) for miR-155 and inhibits c-Myc expression, which has been implicated in the pathogenesis of myeloid leukemia (AML), colorectal cancer, esophageal cancer and lung cancer [33].
Differences of lncRNA expression in recurrent miscarriage
Several studies have demonstrated that lncRNAs are differentially expressed in RM and exhibit tissue-specific expression in embryo sacs (Table 1). A total of 4421 lncRNAs were quantitatively detected in early PCR experiments as having differential expression in chorionic villi, of which 1537 were upregulated, 2884 were downregulated [34]. Meanwhile, 6771 lncRNAs were differentially expressed in the maternal decidua, of these, 3154 lncRNAs were upregulated, and 3617 were downregulated, indicating that differential expression of lncRNAs is more significant in the decidua than in villi [34]. Moreover, Wang et al. [35] identified 1449 differentially expressed lncRNAs (467 upregulated lncRNAs and 982 downregulated lncRNAs) in chorionic villi of RM patients compared with healthy women. And, KEGG pathway analysis revealed that these upregulated and downregulated lncRNAs might target 26 pathways that correspond to transcripts, including 11 upregulated and 15 downregulated pathways [35]. The upregulated lncRNAs participate in the steroid hormone biosynthesis, fatty acid metabolism, glycerophospholipid metabolism, RNA polymerase, ecm receptor interaction process, and the downregulated lncRNAs participate in androgen and oestrogen metabolism, galactose metabolism, purine metabolism, RNA polymerase, glycosphingolipid biosynthesis, indicating that lncRNAs may participate in the pathogenesis of RM by affecting maternal endocrine homeostasis [35]. Furthermore, KEGG pathway analysis also demonstrated that differentially expressed lncRNAs participate in immune-related pathways, indicating that they may regulate the pathogenesis of RM. A critical pathway affected by the upregulation of lncRNAs is the extracellular matrix (ECM) receptor interaction, and GO analysis showed that most lncRNAs are involved in binding and molecular interactions [35]. The endocrine, immune, ECM receptor interaction and apoptosis pathways are the main mechanisms that participate in the pathogenesis of RM. Therefore, it is tempting to speculate that the differentially expressed lncRNAs affect cell adhesion through ECM receptor interactions in the placenta of patients with RM. However, only a few of the mechanisms of lncRNAs have been identified in current studies, and the underlying mechanisms of most other lncRNAs need to be explored (Fig. 1).
Table 1.
Differences of lncRNA expression in RM
| Sample | Method | Upregulated | Downregulated | Refs |
|---|---|---|---|---|
| Chorionic villi | Microarray qRT-PCR | 1537 lncRNAs | 2884 lncRNAs | [34] |
| Maternal decidua | Microarray qRT-PCR | 3154 lncRNAs | 3617 lncRNAs | [35] |
| Chorionic villi | Microarray qRT-PCR | 467 lncRNAs | 982 lncRNAs | [35] |
LncRNA long non‑coding RNA, RM recurrent miscarriage
Fig. 1.
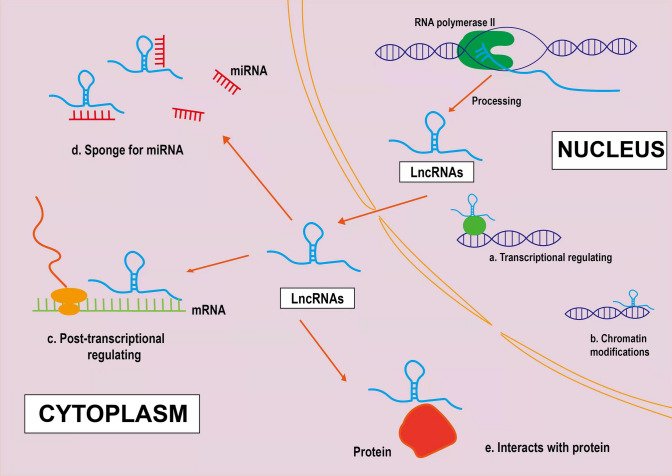
Biogenesis and biological roles of long non-coding RNAs (lncRNAs). LncRNAs are mainly transcribed by RNA polymerase II and have an mRNA-like structure, typically with a 7mC cap at the 5′ end and a polyA tail at the 3′ end. a Some lncRNAs promote or suppress gene expression at transcriptional levels. b Some lncRNAs regulate gene expression by assembling chromatin-modifying complexes. c lncRNAs located in the cytoplasm participate in posttranscriptional gene regulation. d lncRNAs can act as miRNA sponges. e lncRNAs interact with proteins
The mechanism of lncRNAs in recurrent miscarriage
Susceptibility
Susceptibility refers to the degree of susceptibility of humans or animals to infection by a particular pathogen [36], and the genetic material determines the individual's risk of disease. It can also be understood as the risk of different individuals being infected in the same environment. Susceptibility is genetically determined, and genes often play a more critical role under the influence of pathogenic environmental factors [36]. Numerous studies indicated that genetic variation in genes that regulate cellular biological behaviors may be linked to susceptibility to RM (Table 2).
Table 2.
Underlying mechanisms of RM and their associated lncRNAs
| Classification | Underlying mechanism | Associated lncRNA | Refs |
|---|---|---|---|
| 1 | Susceptibility | HULC,CCAT2,MALAT1 | [37–42] |
| 2 | Endometrial receptivity | H19,TUNAR,CECR3, ST7-OT3, DHRS4-AS1, C22orf34, RAMP2-AS1, PNCT-HSA157732 | [43–45] |
| 3 | The maternal-foetal interface: Cellular level | lnc-49a,ANRIL,SLC4A1-1,HOTAIR,HZ08,HZ01 | [46–53] |
| 4 | The maternal-foetal interface: Organizational level | H19 | [54, 55] |
lncRNA long non‑coding RNA, HULC highly upregulated in liver cancer, CCAT2 Colon cancer-associated transcript 2, MALAT1 metastasis associated lung adenocarcinoma transcript-1, TUNAR TCL1 upstream neural differentiation-associated RNA, HOTAIR HOX antisense intergenic RNA
HULC
The HULC (highly up-regulated in liver cancer) gene is located on chromosome 6p24.3, approximately 500 nt in length, promoting different cell phenotypes, including proliferation, survival, and invasion in vivo [56, 57].
Several studies demonstrated that susceptibility to diverse diseases is related to HULC genetic polymorphisms [58, 59]. Fang et al. [37] screened four SNPs in the HULC gene and determined that variant genotypes of rs1041279 C > G, rs17144343 G > A and rs7770772 G > C were linked to a reduced risk of RM, which demonstrated that the rs17144343 GA/AA allele, rs7770772 GC/CC alleles, rs1041279 GG alleles of the HULC gene could decrease RM susceptibility and protect patients against abortion. Meanwhile, it is shown that overexpression of HULC can enhance cells proliferation, invasion and migration without changing the mesenchymal stem cells typing and differentiation abilities [38]. Taken together, lncRNA HULC may also modulate RM susceptibility by altering the biological processes of cells. Yet further research is still required (Fig. 2).
Fig.2.
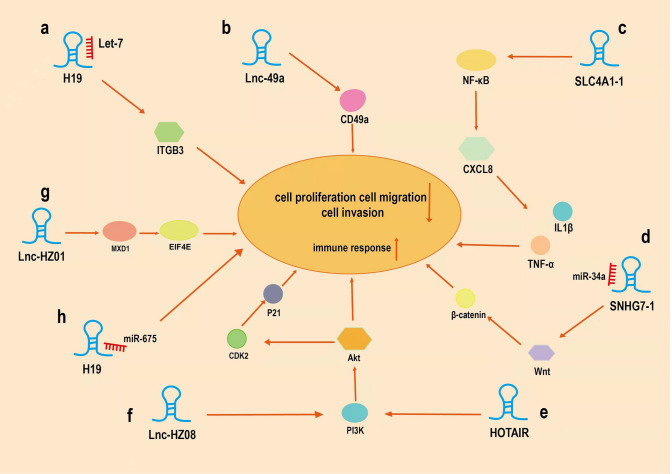
Underlying mechanisms of some lncRNAs in recurrent miscarriage (RM). Some lncRNAs play crucial roles in RM by sponging miRNAs or interacting with other proteins. a H19 interacts with miRNAs of Let-7 to regulate the transcription and translation of integrin β3. b lnc-49a can positively regulate CD49a expression. c lnc-SLC4A1-1 recruit NF-κB and bind to the CXCL8 promoter, which leads to upregulation of CXCL8. d lncRNA SNHG7 interacts with miR-34a to regulate the progression of RM through the Wnt/β-catenin signaling pathway. e lncRNA HOTAIR activates the PI3K-AKT signaling pathway to promote MMP-2 expression. f overexpression lnc-HZ08 suppressed the PI3K and AKT protein levels, and the downstream proteins CDK2 and p-P21. g lnc-HZ01 promotes the transcription of MXD1 mRNA, which promotes the transcription of METTL14 mRNA. h H19 interacts with miR675 to regulate the progression of RM.
CCAT2
CCAT2 (Colon cancer-associated transcript 2) is located on chromosome 8q24 with a 1752 bp lncRNA, which was initially identified in colorectal cancer [60, 61]. Bertucci et al. [39] indicated that the CCAT2 rs6983267 polymorphism is a risk factor for the development of inflammatory breast cancer. Meanwhile, studies have identified that the incidence of RM is associated with inflammation. Che et al. [40] compared the relationship between the polymorphisms of CCAT2 and the susceptibility to RM in 248 patients with RM and 392 healthy patients, and the result confirmed that the CCAT2 rs6983267 G allele is related to a reduced risk of RM. This demonstrates that the variant of rs6983267 G may serve an important role in minimizing the number of RM patients.
MALAT1
MALAT1 (Metastasis associated in lung adenocarcinoma transcript 1) is located on chromosome 11q13 with an 8.5 kb lncRNA, which was initially discovered in early-stage non-small cell lung cancer [62].
It has been confirmed that the MALAT1 expression level is reduced in villus samples of RM patients, and the regulation of MALAT1 is a contributing factor to RM pathogenesis, suggesting that the polymorphism of MALAT1 gene may be related to RM [41]. Furthermore, Che [42] et al. explored the association between MALAT1 gene polymorphism (rs619586) and the combined effects of RM susceptibility and protective genotypes according to age and number of miscarriages. The AG/GG variant is more protective in women under 35 years old and in women who have experienced 2 or 3 miscarriages, compared with the rs619586 AA variant [42]. However, the sample size of these studies was relatively small. Furthermore, larger research samples and additional experimental methods should be used to explore the specific role of lncRNAs in RM, which will be beneficial to determine the cause of RM.
Endometrial receptivity
The endometrium is a layer of the inner wall of the uterus that changes periodically with changes in oestrogen and progesterone [63]. Cyclical changes in the endometrium provide it with a specific ability, endometrial receptivity, which is the ability of the endometrium to accept embryos [64]. In each menstrual cycle, embryos can only be accepted by the endometrium during the implantation window. After the fertilized egg reaches the endometrial cavity, it penetrates the epithelium of the endometrial surface through interactions with long mucin molecules (adherence phase) [65]. Then, the endometrial adhesion molecule αv/β3 integrin (β3) (adhesion phase) tightly adheres to the surface of the endometrium [66]. These biological processes provide adequate preparation for embryonic development and are indispensable for a normal pregnancy.
H19
H19 is located within chromosome 11p15.5 and is expressed in most cells, exclusively by the maternal allele [67]. It has been confirmed that the receptivity of the endometrium during the implantation window decreases when integrin β3 expression decreases [68]. Zeng et al. [43] found that Let-7, as a molecular sponge, is adsorbed by lncRNA H19 to regulate the transcription and translation of integrin β3 (ITGB3) (Table.3). Decreased expression of lncRNA H19 and integrin β3 can be detected in patients with RM, and this process is positively correlated with RM, which reduces endometrial receptivity [43].
Table 3.
lncRNA expression, targets and effects on RM
| lncRNA | Expression | Target | Effect | Refs |
|---|---|---|---|---|
| H19 | Downregulated | miRNA let-7/ITGB3 | Inhibits the adhesion and invasion of HTR-8 cell | [43] |
| Lnc-49a | Downregulated | CD49a | Inhibits the Migration, adhesion, and cytotoxic Activity of dNK cells | [46] |
| Lnc-SLC4A1-1 | Upregulated | NF-κB/CXCL8 | Induces immune responses in trophoblast cells | [47] |
| LncRNA SNHG7-1 | Downregulated | miR-34a/WNT1 | Inhibits proliferation and invasion of trophoblast cells | [48] |
| LncRNA HOTAIR | Downregulated | PIK3-AKT signalling pathway | Inhibits the migration and invasion of trophoblast cells | [49] |
| Lnc-HZ08 | Upregulated | PI3K/p-AKT/P21/CDK2 pathway | Inhibits proliferation, migration, and invasion of trophoblast cells | [52] |
| Lnc-HZ01 | Upregulated | MXD1/METTL14 | Inhibits proliferation of trophoblast cells | [53] |
| H19 | Downregulated | miR-675 | Inhibits proliferation of trophoblast cells | [54] |
lncRNA long non‑coding RNA, ITGB3 inhibit integrin β3, HOTAIR HOX antisense intergenic RNA, CXCL8 C-X-C motif ligand 8, WNT1 Wnt Family Member 1, METTL14 Methyltransferase-like 14
TUNAR
TUNAR (TCL1 Upstream Neural Differentiation-Associated RNA) is an approximately 1.0 kb lncRNA expressed explicitly in the human central nervous system and affects cell differentiation, proliferation, and apoptosis [69, 70]. A study indicated that endometrial biopsies of the late proliferative phase were collected from patients with or without recurrent implantation failure, and the results showed luteinizing hormone (LH) levels of + 2 and + 7 [44]. Meanwhile, the TUNAR expression level in endometrium was downregulated in patients with LH levels of + 7 and upregulated in RM patients. Multiple functions of TUNAR in endometrial epithelial cells (EECs) and endometrial stromal cells (ESCs) were investigated after transfection with pZW1-snoVector-TUNAR [44]. Wang et al. [44] showed for the first time that lncRNA TUNAR was expressed in the human endometrium and might be implicated in embryo implantation by regulating attachment of blastocyst to the endometrial epithelium and modulating the decidualization and proliferation of ESCs. Collectively, TUNAR may play a vital role in regulating endometrial receptivity.
Others
Feng et al. [45] collected 16 mid-luteal endometrial samples (including 8 from the experimental group of patients with RM and 8 from the control group with successful conception) and performed RT-PCR experiments, which demonstrated that the expression of lncRNA CECR3, ST7-OT3, DHRS4-AS1, C22orf34, RAMP2-AS1, and PNCT_HSA157732 were increased significantly in the endometrium of RM patients. Additionally, GO and KEGG pathway functional enrichment analyses confirmed that these six lncRNAs were associated with vascular proliferation, growth factor binding, immune activity, apoptosis, and synthesis of steroid hormones in the uterus to prepare the endometrium for embryo implantation [45]. The above studies indicated that lncRNAs could be used as predictive biomarkers of endometrial receptivity. However, the specific mechanism of lncRNAs remains a significant focus of research.
The maternal-foetal interface
Cellular level
The maternal–fetal interface refers to the endometrium and extra terminal tissue during pregnancy, which is the area where the mother comes in direct contact with the fetus [71]. The maternal–fetal interface consists of decidual immune cells, decidual stromal cells, and trophoblasts [72].
Most immune cells in the decidua, which is crucial to pregnancy, belong to the NK family. NK cells in the decidua are different from killer cells in the traditional sense. Nonetheless, they are “trophoblast” cells that produce many cytokines without the central defensive toxicity of pbNK cells [73]. Although cytotoxic proteins are expressed in dNK cells, including granulysin, granzymes A and B, and perforin providing them cytolytic capacity, this cytotoxic machinery does not cause death of the invading trophoblast except potentially when responding to infection [74]. The cytotoxicity they display is reduced, which may be attributed to the mode of inhibition and activation of receptors expressed on the surface of dNK cells [75].
In addition, trophoblasts arise from the embryonic ectoderm, consisting of syncytiotrophoblasts and cytotrophoblasts. It is essential for pregnancy that trophoblasts invade the uterus. The formation of villous vessels in early pregnancy promotes the invasion of trophoblasts. If the formation of villous vessels is blocked, the invasion of trophoblasts will be obstructed, which will eventually lead to abortion. It has been shown that the migration and invasion of trophoblasts are associated with complex biochemical interactions, including increasing cell adhesion and enhancing cell proliferation [76].
Lnc-49a
Li et al. [46] examined the deciduae of 15 groups of patients with recurrent abortion and 15 groups of patients with normal abortion, which found that lnc-49a can positively regulate CD49a expression and maintain reduced cytotoxic activity. The adhesion and migration of dNK cells were downregulated, while the expression levels of interferon-γ granzyme B, and perforin in dNK cells were upregulated by a CD49a-neutralizing antibody which increased the killing ability of dNK cells [46]. It can be concluded that Lnc-49a can alter the homeostasis of the decidual microenvironment, leading to recurrent spontaneous abortion.
ANRIL
ANRIL (the long antisense non‐coding RNA at the INK4 locus) is located within chromosome 9p21, approximately 3.8 kb in length [77]. LncRNA ANRIL and mVEGF expression levels in villi of RM patients were positively correlated, and both were down-regulated, suggesting that lncRNA ANRIL may be down-regulated and may further inhibit villous vascular formation and trophoblast invasion in patients with RM by regulating the down-regulation of VEGF expression.
Lnc-SLC4A1-1
Lnc-SLC4A1-1 has been demonstrated to recruit NF-κB and bind to the CXCL8 promoter, which contributes to upregulation of CXCL8 [47]. Meanwhile, the elevation of CXCL8 intensifies the inflammatory response by inducing TNF-α and IL-1β, which can lead to the apoptosis of trophoblasts [47]. This finding represents a new step in determining the mechanism of recurrent spontaneous abortion.
SNHG7
SNHG7 (small nucleolar RNA host gene 7) is a known lncRNAs, with a total length of 2176 bp, which is located within chromosome 9q34 [78]. Research has indicated that abnormal expression of the Wnt/β-catenin pathway may be involved in RM. Xiang et al. [48] used qRT-PCR to determine that lncRNA SNHG7-1 was downregulated in HTR-8/SVneo cells. Knockdown of lncRNA SNHG7 can inhibit the proliferation, invasion and induce apoptosis in HTR-8/SVneo cells. When miR-34a was overexpressed in HTR-8/SVneo cells, WNT1 expression was significantly downregulated because miR-34a inhibits WNT1 expression. Hence, targeting lncRNA SNHG7 by miR-34a plays a vital role in the progression of RM through the Wnt/β-catenin signaling pathway, providing valuable therapeutic targets for patients with RM.
HOTAIR
HOTAIR (HOX transcript antisense RNA) is approximately 2.2 kb in length, which is located in chromosome 12q13 [79]. Zhang et al. [49] found that YY1, as a transcriptional activator of lncRNA HOTAIR, activates the PI3K-AKT signaling pathway to promote MMP-2 expression by enhancing the migration and invasion of trophoblasts. In another study, Wang et al. [50] separated primary villous trophoblasts to examine the P53-MALAT1 axis, showing that P53 expression was upregulated in the villi of RM patients and the lncRNA MALAT1 expression level was downregulated. Moreover, P53 is a negative regulator that has a vital role in many biological processes, including the cell cycle, apoptosis and differentiation [51]. Upregulation of P53 expression promotes cell apoptosis and reduces the survival rate of trophoblasts, leading to RM.
Lnc-HZ08
Increasing evidence has indicated that pregnant women might miscarry after exposure to environmental benzo(a)pyrene(BaP). Additionally, benzo(a)pyren-7,8-dihydrodiol-9,10-epoxide(BPDE), the ultimate metabolite of BaP, could induce dysfunction in trophoblasts. The expression of lnc-HZ08 was significantly upregulated in both RM tissue and BPDE-treated cells, and overexpression lnc-HZ08 suppressed the PI3K and AKT protein levels, and the downstream proteins CDK2 and p-P21 [52]. Meanwhile, lnc-HZ08 promoted the ubiquitin degradation of PI3K by promoting the interaction between CBL and PI3K in trophoblasts; therefore, lnc-HZ08 may negatively regulate proliferation, invasion and migration by inhibiting the PI3K/p-AKT/P21/CDK2 signaling pathway in BPDE-exposed trophoblasts [52].
Lnc-HZ01
In trophoblasts, lnc-HZ01 promotes the transcription of MXD1 mRNA by upregulating c-JUN, promotes stability of MXD1 by upregulating its deubiquitinating enzyme USP36, and eventually upregulates the level of MXD1 protein in trophoblasts nucleus [53]. However, MXD1 promotes the transcription of METTL14 mRNA and upregulates the level of lnc-HZ01 m6A RNA methylation, which promotes the stability of lnc-HZ01 and increases its expression level. Therefore, lnc-HZ01 and MXD1 upregulate each other, forming a positive self-feedback loop. Meanwhile, BPDE could activate this loop by upregulating the MXD1/METTL14/lnc-HZ01 and lnc-HZ01/MXD1 signaling pathways. Once the loop is activated by BPDE exposure, EIF4E expression levels are upregulated, and the proliferation of trophoblasts is suppressed, which eventually leads to miscarriage [53]. Therefore, lnc-HZ01 modulates both the proliferation of trophoblasts and the occurrence of miscarriage. Together, the above studies have shown that trophoblasts mainly control RM, and disrupting the function of trophoblasts may lead to RM.
Organizational level
The placenta is an organ that conducts material exchange between the mother and the foetus and is composed of the amniotic membrane, phyllodes chorionic membrane, and decidua basalis. When the fertilized egg is implanted, it adheres to the maternal endometrium and trophoblasts of the embryo invade the endometrium, which eventually results in placentation [72]. Furthermore, normal development of the placenta is vital for a normal pregnancy.
H19
lncRNA H19 is the template of miR-675. The stem-loop of miR-675 has been indicated to be one of the most conserved features of H19 RNA in the evolution of mammals. Examination of the embryonic tissues of 43 RM patients and 55 control patients demonstrated that the expression level of lncRNA H19 was upregulated in RM patients. Liu et al. [54] used H19-silenced JEG-3 cells that were transiently transfected with siRNA to detect the downregulation of miR-675 expression. When the miR-675 expression is downregulated, the growth of the placenta will also be inhibited. Further experiments confirmed that NOMO1 is a target gene that regulates H19/miR-675 in human trophoblasts [54]. Intriguingly, another studies have shown that lncRNA H19 slows placental growth in the second trimester of pregnancy by downregulating the RNA-binding protein HuR, which usually hinders miR-675 processing during the Drosha stage [55]. Increased expression of miR-675 in the placenta is often accompanied by downregulation of lgf1r, which can lead to slower growth, indicating that miR-675 is negatively correlated with placental development. Conclusions drawn from the role of lncRNA H19 in RM should not be ignored because they will provide a solid foundation for treatment.
Future perspectives
In recent years, studies of lncRNAs have made breakthroughs, but due to the abundance of lncRNAs, these studies only provide a small amount of information, and many functions of lncRNAs have not been explored. In addition, the vast majority of the thousands of mammalian lncRNAs that have been identified thus far remain entirely uncharacterized. Therefore, the full mechanisms by which these molecules regulate biological processes remain unknown. LncRNAs have a wide range of functions and are involved in regulating the occurrence and development of many diseases, which is the main reason why they have become a focus of research in recent years. Existing experimental studies have a significant flaw because most lncRNA functions are explored at the cellular level, while in vivo animal experiments are rarely carried out. Designing animal experiments to explore the functions and mechanisms of lncRNAs is a problem that urgently needs to be solved. Experimental methods and tools for the study of lncRNAs will be developed in the future, which will provide a theoretical basis for the diagnosis and treatment of RM.
Acknowledgements
Not applicable.
Abbreviations
- CCAT2
Colon cancer-associated transcript 2
- CXCL8
C-X-C motif ligand 8
- HOTAIR
HOX antisense intergenic RNA
- HULC
Highly upregulated in liver cancer
- ITGB3
Inhibit integrin β3
- lncRNA
Long non‑coding RNA
- MALAT1
Metastasis associated lung adenocarcinoma transcript-1
- METTL14
Methyltransferase-like 14
- RM
Recurrent miscarriage
- TUNAR
TCL1 upstream neural differentiation-associated RNA
- WNT1
Wnt family member 1
Author contributions
Conceptualization, S.W.; data curation, S.W. and Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, S.W.; visualization, Y.Z.; supervision, S.W.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the National Natural Science Foundation of China (81741038 and 81100447), the Natural Science Foundation of Shandong Province(ZR2016HM10), and the Jinan Science and Technology Program(202134014 and 201907012).
Data availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Practice Committee of the American Society for Reproductive Medicine Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;89(6):1603. doi: 10.1016/j.fertnstert.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Hertz-Picciotto I, Samuels SJ. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(22):1483–1484. doi: 10.1056/NEJM198812013192214. [DOI] [PubMed] [Google Scholar]
- 3.Gibbins KJ, Porter TF. The importance of an evidence-based workup for recurrent pregnancy loss. Clin Obstet Gynecol. 2016;59(3):456–463. doi: 10.1097/GRF.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HY, Park HS, Ko EJ, et al. Association of complement factor D and H polymorphisms with recurrent pregnancy loss. Int J Mol Sci. 2019;21(1):17. doi: 10.3390/ijms21010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Practice Committee of the American Society for Reproductive Medicine Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99:63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Bilibio JP, Gama TB, Nascimento ICM, et al. Causes of recurrent miscarriage after spontaneous pregnancy and after in vitro fertilization. Am J Reprod Immunol. 2020;83(5):e13226. doi: 10.1111/aji.13226. [DOI] [PubMed] [Google Scholar]
- 7.Heo MJ, Yun J, Kim SG. Role of non-coding RNAs in liver disease progression to hepatocellular carcinoma. Arch Pharm Res. 2019;42(1):48–62. doi: 10.1007/s12272-018-01104-x. [DOI] [PubMed] [Google Scholar]
- 8.Andersen RE, Lim DA. Forging our understanding of lncRNAs in the brain. Cell Tissue Res. 2018;371(1):55–71. doi: 10.1007/s00441-017-2711-z. [DOI] [PubMed] [Google Scholar]
- 9.Isin M, Dalay N. LncRNAs and neoplasia. Clin Chim Acta. 2015;444:280–288. doi: 10.1016/j.cca.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Yin Y, Yan P, Lu J, et al. Opposing roles for the lncRNA haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. Cell Stem Cell. 2015;16(5):504–516. doi: 10.1016/j.stem.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Blokhin I, Khorkova O, Hsiao J, et al. Developments in lncRNA drug discovery: where are we heading. Expert Opin Drug Discov. 2018;13(9):837–849. doi: 10.1080/17460441.2018.1501024. [DOI] [PubMed] [Google Scholar]
- 12.Guzel E, Okyay TM, Yalcinkaya B, et al. Tumor suppressor and oncogenic role of long non-coding RNAs in cancer. North Clin Istanb. 2019;7(1):81–86. doi: 10.14744/nci.2019.46873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tornesello ML, Faraonio R, Buonaguro L, et al. The role of microRNAs, long non-coding RNAs, and circular RNAs in cervical cancer. Front Oncol. 2020;10:150. doi: 10.3389/fonc.2020.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaux Y, Zangrando J, Schroen B, et al. Long noncoding RNAs in cardiac development and ageing. Nat Rev Cardiol. 2015;12(7):415–425. doi: 10.1038/nrcardio.2015.55. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Hu H, Yan G, et al. Long non-coding RNA and breast cancer. Technol Cancer Res Treat. 2019;18:1533033819843889. doi: 10.1177/1533033819843889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batagov AO, Yarmishyn AA, Jenjaroenpun P, et al. Role of genomic architecture in the expression dynamics of long noncoding RNAs during differentiation of human neuroblastoma cells. BMC Syst Biol. 2013 doi: 10.1186/1752-0509-7-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jathar S, Kumar V, Srivastava J, et al. Technological developments in lncRNA biology. Adv Exp Med Biol. 2017;1008:283–323. doi: 10.1007/978-981-10-5203-3_10. [DOI] [PubMed] [Google Scholar]
- 21.Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Marinov GK, Pepke S, et al. Single-cell transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. Cell Stem Cell. 2015;16(1):88–101. doi: 10.1016/j.stem.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattick JS, Rinn JL, et al. Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol. 2015;22(1):5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Fu H, Wu Y, et al. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci. 2013;56(10):876–885. doi: 10.1007/s11427-013-4553-6. [DOI] [PubMed] [Google Scholar]
- 25.Hombach S, Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Wang W, Zhu W, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 2019;20(22):5573. doi: 10.3390/ijms20225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhade VS, Pal D, Kanduri C. Long noncoding RNA: genome organization and mechanism of action. Adv Exp Med Biol. 2017;1008:47–74. doi: 10.1007/978-981-10-5203-3_2. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425(19):3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Lin C, Jin C, et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung CL, Wang LY, Yu YL, et al. A long noncoding RNA connects c-Myc to tumor metabolism. Proc Natl Acad Sci U S A. 2014;111:18697–18702. doi: 10.1073/pnas.1415669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguilo F, Zhou MM, Walsh MJ. Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res. 2011;71:5365–5369. doi: 10.1158/0008-5472.CAN-10-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Hua Y. CCAT1: an oncogenic long noncoding RNA in human cancers. J Cancer Res Clin Oncol. 2017;143:555–562. doi: 10.1007/s00432-016-2268-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Cao Q, Ge J, et al. LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: genome wide differential expression of lncRNAs in early spontaneous abortion. Am J Reprod Immunol. 2014;72(4):359–375. doi: 10.1111/aji.12275. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Tang H, Xiong Y, et al. Differential expression profile of long noncoding RNAs in human chorionic villi of early recurrent miscarriage. Clin Chim Acta. 2017;464:17–23. doi: 10.1016/j.cca.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Boyce WT. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology. 2016;41(1):142–162. doi: 10.1038/npp.2015.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang Z, Yang Y, Xu Y, et al. LncRNA HULC polymorphism is associated with recurrent spontaneous abortion susceptibility in the Southern Chinese population. Front Genet. 2019;10:918. doi: 10.3389/fgene.2019.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Wang J, Pan Y, et al. Long non-coding RNA HULC affects the proliferation, apoptosis, migration, and invasion of mesenchymal stem cells. Exp Biol Med (Maywood) 2018;243(13):1074–1082. doi: 10.1177/1535370218804781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertucci F, Lagarde A, Ferrari A, et al. 8q24 Cancer risk allele associated with major metastatic risk in inflammatory breast cancer. PLoS One. 2012;7(5):e37943. doi: 10.1371/journal.pone.0037943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Che D, Huang W, Fang Z, et al. The lncRNA CCAT2 rs6983267 G allele is associated with decreased susceptibility to recurrent miscarriage. J Cell Physiol. 2019;234(11):20577–20583. doi: 10.1002/jcp.28661. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Liu HZ, Liu Y, et al. Downregulated MALAT1 relates to recurrent pregnancy loss via sponging miRNAs. Kaohsiung J Med Sci. 2018;34(9):503–510. doi: 10.1016/j.kjms.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Che D, Yang Y, Xu Y, et al. The lncRNA MALAT1 rs619586 G variant confers decreased susceptibility to recurrent miscarriage. Front Physiol. 2019;10:385. doi: 10.3389/fphys.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He D, Zeng H, Chen J, et al. H19 regulates trophoblastic spheroid adhesion by competitively binding to let-7. Reproduction. 2019;157(5):423–430. doi: 10.1530/REP-18-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Hu S, Yao G, et al. A novel molecule in human cyclic endometrium: lncRNA TUNAR is involved in embryo implantation. Front Physiol. 2020;11:587448. doi: 10.3389/fphys.2020.587448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng C, Shen JM, Lv PP, et al. Construction of implantation failure related lncRNA-mRNA network and identification of lncRNA biomarkers for predicting endometrial receptivity. Int J Biol Sci. 2018;14(10):1361–1377. doi: 10.7150/ijbs.25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Hou Y, Zhang S, et al. CD49a regulates the function of human decidual natural killer cells. Am J Reprod Immunol. 2019;81(4):e13101. doi: 10.1111/aji.13101. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z, Du G, Huang X, et al. The enhancer RNA lnc-SLC4A1-1 epigenetically regulates unexplained recurrent pregnancy loss (URPL) by activating CXCL8 and NF-kB pathway. EBioMedicine. 2018;38:162–170. doi: 10.1016/j.ebiom.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang H, Yan H, Sun B, et al. Decreased expression of long non-coding RNA SNHG7 cause recurrent spontaneous abortion through suppression proliferation and invasion of trophoblast cells via miR-34a. Am J Transl Res. 2019;11(1):463–472. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Jin F, Li XC, et al. The YY1-HOTAIR-MMP2 signaling axis controls trophoblast invasion at the maternal-fetal interface. Mol Ther. 2017;25(10):2394–2403. doi: 10.1016/j.ymthe.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Wang Y, Liu HZ, Liu Y, et al. Disordered p53-MALAT1 pathway is associated with recurrent miscarriage. Kaohsiung J Med Sci. 2019;35(2):87–94. doi: 10.1002/kjm2.12013. [DOI] [PubMed] [Google Scholar]
- 51.Bauer JH, Helfand SL. New tricks of an old molecule: lifespan regulation by p53. Aging Cell. 2006;5:437–440. doi: 10.1111/j.1474-9726.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie J, Liang T, Zhao J, et al. Lnc-HZ08 regulates BPDE-induced trophoblast cell dysfunctions by promoting PI3K ubiquitin degradation and is associated with miscarriage. Cell Biol Toxicol. 2021 doi: 10.1007/s10565-021-09606-z. [DOI] [PubMed] [Google Scholar]
- 53.Xu Z, Tian P, Guo J, et al. Lnc-HZ01 with m6A RNA methylation inhibits human trophoblast cell proliferation and induces miscarriage by up-regulating BPDE-activated lnc-HZ01/MXD1 positive feedback loop. Sci Total Environ. 2021;776:145950. doi: 10.1016/j.scitotenv.2021.145950. [DOI] [PubMed] [Google Scholar]
- 54.Gao WL, Liu M, Yang Y, et al. The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets nodal modulator 1 (NOMO1) RNA Biol. 2012;9(7):1002–1010. doi: 10.4161/rna.20807. [DOI] [PubMed] [Google Scholar]
- 55.Keniry A, Oxley D, Monnier P, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14(7):659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Guo Q, Chen J, et al. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep. 2014;31(1):358–364. doi: 10.3892/or.2013.2850. [DOI] [PubMed] [Google Scholar]
- 57.Cui M, Xiao Z, Wang Y, et al. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res. 2015;75(5):846–857. doi: 10.1158/0008-5472.CAN-14-1192. [DOI] [PubMed] [Google Scholar]
- 58.Wang BG, Lv Z, Ding HX, et al. The association of lncRNA-HULC polymorphisms with hepatocellular cancer risk and prognosis. Gene. 2018;670:148–154. doi: 10.1016/j.gene.2018.05.096. [DOI] [PubMed] [Google Scholar]
- 59.Shaker OG, Senousy MA, Elbaz EM. Association of rs6983267 at 8q24, HULC rs7763881 polymorphisms and serum lncRNAs CCAT2 and HULC with colorectal cancer in Egyptian patients. Sci Rep. 2017;7(1):16246. doi: 10.1038/s41598-017-16500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao P, Sun D, Guo H, et al. LncRNA CCAT2 promotes proliferation and suppresses apoptosis of colorectal cancer cells. J BUON. 2020;25(4):1840–1846. [PubMed] [Google Scholar]
- 61.Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23(9):1446–1461. doi: 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 63.Critchley HOD, Maybin JA, Armstrong GM, et al. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;100(3):1149–1179. doi: 10.1152/physrev.00031.2019. [DOI] [PubMed] [Google Scholar]
- 64.Kelleher AM, Milano-Foster J, Behura SK, et al. Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat Commun. 2018;9(1):2435. doi: 10.1038/s41467-018-04848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meseguer M, Aplin JD, Caballero-Campo P, et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001;64(2):590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 66.Lessey BA, Damjanovich L, Coutifaris C, et al. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188–195. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: roles in tumorigenesis. Biomed Pharmacother. 2020;123:109774. doi: 10.1016/j.biopha.2019.109774. [DOI] [PubMed] [Google Scholar]
- 68.Germeyer A, Savaris RF, Jauckus J, et al. Endometrial beta3 integrin profile reflects endometrial receptivity defects in women with unexplained recurrent pregnancy loss. Reprod Biol Endocrinol. 2014;12:53. doi: 10.1186/1477-7827-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou AX, Mondal T, Tabish AM, et al. The long noncoding RNA TUNAR modulates Wnt signaling and regulates human β-cell proliferation. Am J Physiol Endocrinol Metab. 2021;320(4):E846–E857. doi: 10.1152/ajpendo.00335.2020. [DOI] [PubMed] [Google Scholar]
- 70.Li M, Shao F, Qian Q, et al. A putative long noncoding RNA-encoded micropeptide maintains cellular homeostasis in pancreatic β cells. Mol Ther Nucleic Acids. 2021;26:307–320. doi: 10.1016/j.omtn.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y, Plazyo O, Romero R, et al. Isolation of leukocytes from the human maternal-fetal interface. J Vis Exp. 2015;99:e52863. doi: 10.3791/52863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75(3):341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 73.Bachmayer N, Rafik Hamad R, Liszka L, et al. Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am J Reprod Immunol. 2006;56:292–301. doi: 10.1111/j.1600-0897.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 74.Blois SM, Klapp BF, Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol. 2011;88:86–92. doi: 10.1016/j.jri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18(4):458–471. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbas Y, Turco MY, Burton GJ, et al. Investigation of human trophoblast invasion in vitro. Hum Reprod Update. 2020;26(4):501–513. doi: 10.1093/humupd/dmaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L, Qu H, Guo M, et al. ANRIL and atherosclerosis. J Clin Pharm Ther. 2020;45(2):240–248. doi: 10.1111/jcpt.13060. [DOI] [PubMed] [Google Scholar]
- 78.Ota T, Suzuki Y, Nishikawa T, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2004;36(1):40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 79.Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding rnas. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.