Abstract
Trichosporon asahii is a conditional pathogenic fungus that causes severe and sometimes fatal infections in immunocompromised patients. While calcineurin, an essential component of a calcium-dependent signaling pathway, is known to regulate stress resistance and virulence of some pathogenic fungi, its role in T. asahii has not been investigated. Here, we demonstrated that calcineurin gene-deficient T. asahii mutants are sensitive to high temperature as well as cell-membrane and cell-wall stress, and exhibit decreased hyphal formation and virulence against silkworms. Growth of T. asahii mutants deficient in genes encoding subunits of calcineurin, cna1 and cnb1, was delayed at 40 °C. The cna1 and cnb1 gene-deficient mutants also showed sensitivity to sodium dodecyl sulfate, Congo red, dithiothreitol, and tunicamycin. On the other hand, these mutants exhibited no sensitivity to caffeine, sorbitol, monensin, CaCl2, LiCl, NaCl, amphotericin B, fluconazole, or voriconazole. The ratio of hyphal formation in the cna1 and cnb1 gene-deficient mutants was decreased. Moreover, the virulence of the cna1 and cnb1 gene-deficient mutants against silkworms was attenuated. These phenotypes were restored by re-introducing each respective gene into the gene-deficient mutants. Our findings suggest that calcineurin has a role in regulating the cellular stress response and virulence of T. asahii.
Subject terms: Fungi, Microbial genetics, Pathogens
Introduction
Trichosporon asahii is a basidiomycete yeast widely distributed in the environment and is often isolated from human blood, sputum, skin, feces, and urine1–6. T. asahii causes severe fungal infections in immunocompromised patients7–9, and the mortality rate of deep mycoses caused by T. asahii is twice as high as that caused by Candida albicans (80% vs 40%)10. T. asahii is resistant to echinocandin antifungals, and thus patients treated with micafungin are susceptible to the development of severe infections11. Amphotericin B- and azole antifungal-resistant T. asahii strains have also been isolated from patients12,13. T. asahii commonly forms a biofilm comprising microbe aggregates and extracellular matrix on indwelling medical devices14. The biofilm formation by T. asahii has a function to confer its resistance to antifungal drugs13. Several morphologic forms of T. asahii exist, such as yeast, hyphae (filament form), and arthroconidia (chains of cells and asexual spores)4. Arthroconidia of T. asahii contribute to biofilm formation by promoting cellular adhesion15. These features of T. asahii make it a highly problematic clinical pathogen9.
Calcineurin, a calcium-calmodulin-activated phosphatase consisting of a heterodimer with the catalytic and regulatory subunits Cna1 and Cnb116 controls the expression of several genes by dephosphorylating the transcriptional regulator Crz1 through binding to calmodulin, a calcium sensor17,18. It is essential for the growth of Cryptococcus neoformans, a pathogenic basidiomycete yeast like T. asahii, at 37 °C, as well as its virulence in a rabbit model of cryptococcal meningitis and cation homeostasis19,20. In addition, calcineurin contributes to resistance to cell membrane damage, cell wall damage, osmotic stress, and endoplasmic reticulum (ER) stress21,22. The role of calcineurin in the virulence of T. asahii, however, has remained unclear.
To elucidate the virulence of T. asahii, we used an established silkworm infection model23. Although mammalian experimental models such as mice are usually used in studies of infectious diseases24, the requirements of specialized experimental facilities and large numbers of animals as well as the ethical considerations are severely limiting25. T. asahii infection experiments are also not easy to perform in mice because immunosuppressive drugs must be administered26,27. The use of an invertebrate silkworm model for these types of experiments is highly advantageous because silkworms are less costly to house and rear in large numbers, and fewer ethical problems are associated with their use.
In the present study, we used a recently developed technique28 to generate calcineurin gene-deficient T. asahii mutants and characterized the phenotypes related to stress resistance and virulence in the silkworm infection model. The cna1 and cnb1 gene-deficient mutants exhibited sensitivity to chemicals known to cause membrane damage and ER stress, as well as attenuated hyphal formation and virulence against silkworms. Our findings suggest that calcineurin controls cellular stress responses and virulence of T. asahii via a calcineurin-signaling pathway.
Results
Generation of cna1, cnb1 gene-deficient mutants, and their gene-reintroduced strains
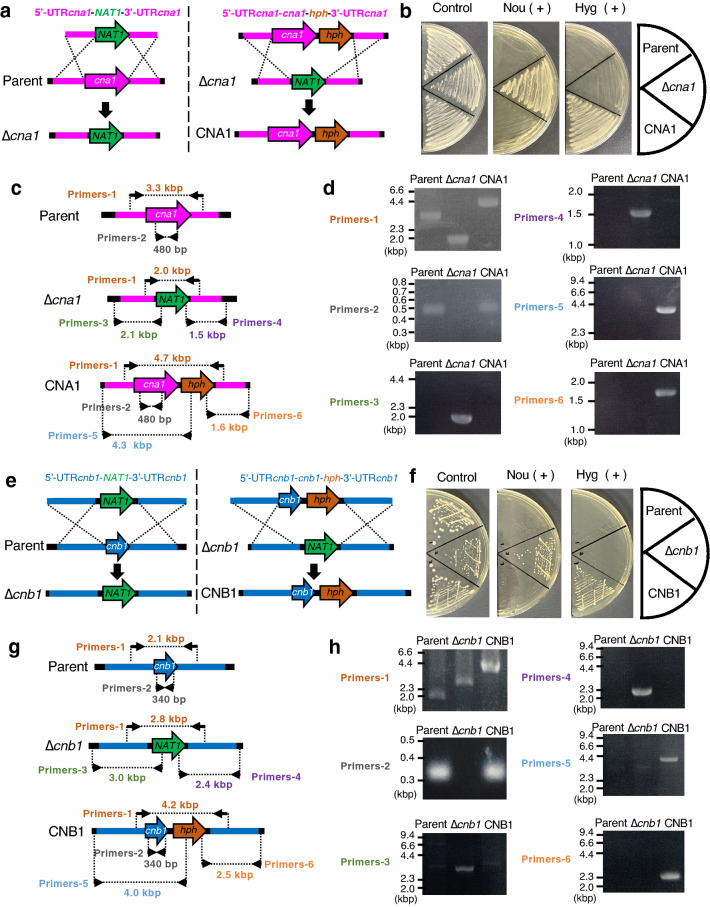
The ku70 gene-deficient strain of the T. asahii MPU129 strain has high homologous recombination efficiency, making it useful as a parent strain28. Using this strain, we generated the cna1 gene-deficient mutant. The targeting DNA fragment used to generate the cna1 gene-deficient mutant contains the NAT1 gene, which leads to nourseothricin resistance (Fig. 1a). Introducing this DNA fragment into the T. asahii genome confers resistance to nourseothricin (Fig. 1a). Nourseothricin-resistant strains were obtained by introducing the DNA fragment through electroporation (Fig. 1b). The genomes were extracted from the transformants and the deficiency of the cna1 gene was checked by polymerase chain reaction (PCR) (Fig. 1c,d). Secondary genetic mutations such as point mutations may occur during the generation of gene-deficient mutants. Therefore, it is necessary to establish a revertant strain in which the gene is reintroduced into the gene-deficient mutant to confirm that the phenotype of the gene-deficient strain is due to a deficiency of the targeted gene. Next, we generated a revertant strain of the cna1 gene-deficient mutant. The targeting DNA fragment used to generate the revertant strain of the cna1 gene-deficient mutant contains the hgh gene, which leads to hygromycin B resistance (Fig. 1a). Hygromycin B-resistant strains were obtained by introducing the DNA fragment through electroporation (Fig. 1b). Reintroduction of the cna1 gene was checked by PCR using the genomes extracted from the transformants (Fig. 1c,d). The results confirmed the generation of the cna1 gene-deficient T. asahii mutant and the cna1 gene-reintroduced revertant. Similarly, a cnb1 gene-deficient mutant and its revertant were also generated (Fig. 1e–h).
Figure 1.
Generation of the cna1 and cnb1 gene-deficient T. asahii mutants and their revertants. (a–d) Generation of the cna1 gene-deficient mutant and its revertant in T. asahii. (a) Strategy for generating the cna1 gene-deficient mutant (∆cna1) and its revertant (Rev.). Predicted genomes of the cna1 gene-deficient mutant and its revertant are shown. (b) The parent strain (Parent), cna1 gene-deficient mutant (∆cna1), and its revertant (Rev.) were spread on SDA with nourseothricin (Nou) (100 µg/ml) or hygromycin B (Hyg) (100 µg/ml) and incubated at 27 °C for 2 days. (c) Location of the primers for confirming the genome structure of the cna1 gene-deficient candidate by PCR using extracted genome DNA. (d) Confirmation of the genotypes of the cna1 gene-deficient mutant (∆cna1) and its revertant (Rev.) by PCR using extracted genome DNA. (e–h) Generation of the cna1 gene-deficient mutant and its revertant in T. asahii. (e) Strategy for generating the cnb1 gene-deficient mutant (∆cnb1) and its revertant (Rev.). Predicted genomes of the cnb1 gene-deficient mutant and its revertant are shown. (f) The parent strain (Parent), cnb1 gene-deficient mutant (∆cnb1), and its revertant (Rev.) were spread on SDA with nourseothricin (Nou) (100 µg/ml) or hygromycin B (Hyg) (100 µg/ml) and incubated at 27 °C for 2 days. (g) Location of the primers for confirming the genome structure of the cnb1 gene-deficient candidate by PCR using extracted genome DNA. (h) Confirmation of the genotypes of the cnb1 gene-deficient mutant (∆cnb1) and its revertant (Rev.) by PCR using extracted genome DNA. Cropped blots were used. Full-length blots are presented in Supplementary Fig. S3.
Function of calcineurin in stress resistance of T. asahii
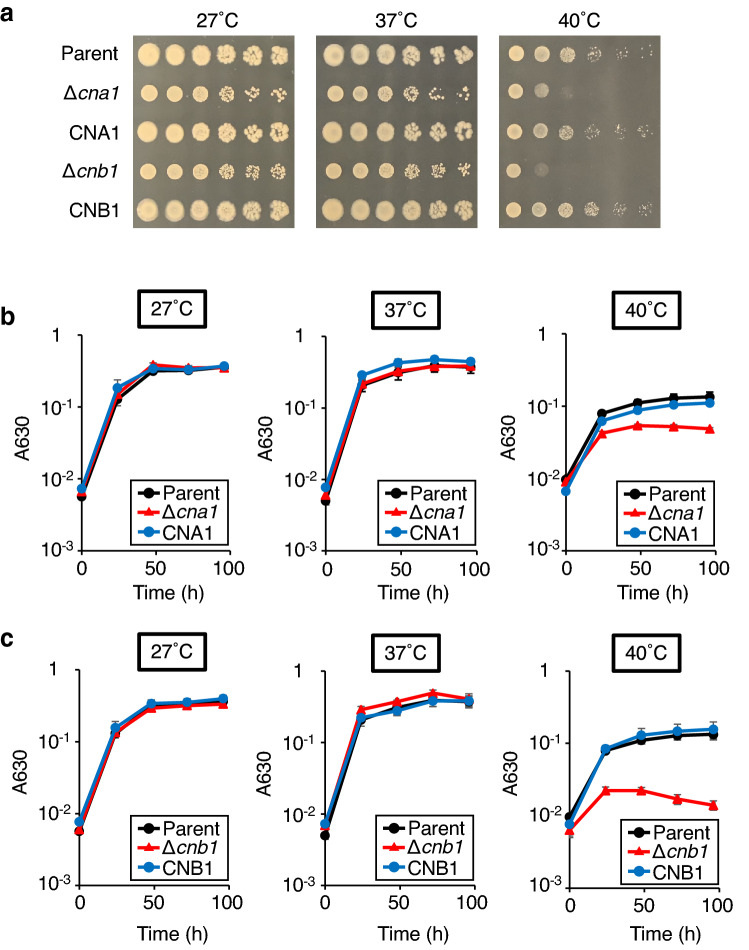
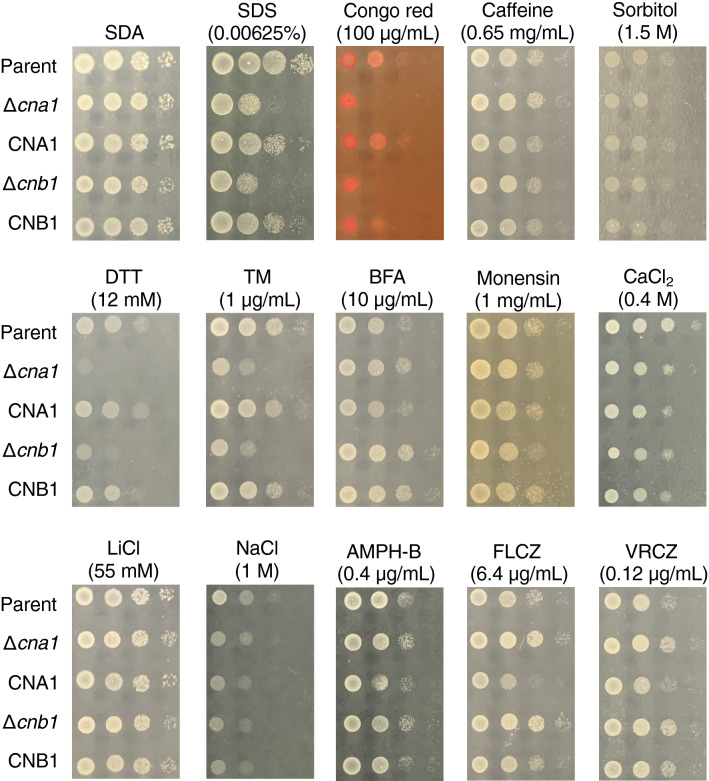
In C. neoformans, cna1 and cnb1 gene-deficient mutants show growth inhibition at 37 °C29,30. Growth of the cna1 and cnb1 gene-deficient T. asahii mutants was delayed at 40 °C (Fig. 2). The high-temperature sensitive phenotype of these gene-deficient mutants was suppressed in their revertants (Fig. 2). In Cryptococcus gattii and C. neoformans, cna1 gene-deficient mutants are sensitive to sodium dodecyl sulfate (SDS), which damages the cell membrane, and Congo red, which damages the cell wall21. The cna1 gene-deficient mutant of C. neoformans is also sensitive to caffeine, which inhibits the cell wall integrity (CWI) signaling pathway, which promotes cell wall integrity; and sorbitol, which is an osmotic stressor22. Growth was delayed in cna1 and cnb1 gene-deficient T. asahii mutants treated with SDS and Congo red, but not affected by treatment with caffeine or sorbitol (Fig. 3). In C. neoformans, the cna1 gene-deficient mutant is sensitive to dithiothreitol (DTT) and tunicamycin (TM), which stress the ER; brefeldin A (BFA), which inhibits intracellular vesicle formation and protein trafficking between the ER and the Golgi apparatus; and monensin, which is involved in intracellular transport22. Growth was delayed in cna1 and cnb1 gene-deficient T. asahii mutants treated with DTT and TM, but not in those treated with BFA and monensin (Fig. 3). The presence of Ca2+ or Li+ inhibits the growth of the cna1 gene-deficient Cryptococcus gattii mutant, but not that of the cna1 gene-deficient C. neoformans mutant21. The growth of cna1 and cnb1 gene-deficient T. asahii mutants was not altered by treatment with CaCl2, LiCl, or NaCl (Fig. 3). Moreover, the presence of antifungal drugs such as amphotericin B, fluconazole, and voriconazole did not cause growth inhibition in the cna1 and cnb1 gene-deficient T. asahii mutants (Fig. 3). The stress-sensitive phenotypes of these gene-deficient mutants were suppressed in the revertants (Fig. 3). These findings suggest that the cna1 and cnb1 genes are involved in resistance to cell membrane and cell wall damage, and ER stress in T. asahii.
Figure 2.
Temperature sensitivity of cna1 or cnb1 gene-deficiency in T. asahii. (a) The T. asahii parent strain (Parent), the cna1 gene-deficient mutant (∆cna1), the revertant of ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant of ∆cnb1 (CNB1) were grown on SDA and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution and filtered through a 40-μm cell strainer. Series of tenfold dilution of the fungal suspension were prepared using saline. Five microliters of each cell suspension was spotted on the SDA. Agar plates were incubated at 27 °C, 37 °C, or 40 °C for 24 h. (b, c) The T. asahii parent strain (Parent), the cna1 gene-deficient mutant (∆cna1), the revertant of ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant of ∆cnb1 (CNB1) were inoculated on Sabouraud medium and incubated at 27 °C, 37 °C, or 40 °C. Absorbance of the culture at 630 nm was monitored. Data are shown as means ± standard error of the mean (SEM).
Figure 3.
Sensitivity of the cna1 and cnb1 gene-deficient mutants against stress inducers. The T. asahii parent strain (Parent), the cna1 gene-deficient mutant (∆cna1), the revertant of ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant of ∆cnb1 (CNB1) were grown on SDA and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution and filtered through a 40-μm cell strainer. Series of tenfold dilution of the fungal suspension were prepared using saline. Five microliters of each cell suspension was spotted on the SDA containing SDS (0.00625%), Congo red (100 µg/ml), caffeine (0.65 mg/ml), sorbitol (1.5 M), DTT (12 mM), TM (1 µg/ml), BFA (10 µg/ml), monensin (1 mg/ml), CaCl2 (0.4 M), LiCl (55 mM), NaCl (1 M), amphotericin-B (0.4 µg/ml), fluconazole (6.4 µg/ml), or voriconazole (0.12 µg/ml). Each agar plate was incubated at 37 °C for 24 h.
In C. neoformans, DTT induces the unfolded protein response (UPR) signaling31. The HXL1 mRNA splicing by Ire1 protein, a key factor of UPR signaling, was increased by DTT in C. neoformans31. Moreover, the HXL1 gene in T. asahii includes the putative unconventional splicing site that is spliced by Ire1 protein32. We tested the effect of DTT on the UPR signaling in T. asahii. The amounts of spliced HXL1 mRNA in T. asahii were increased by treating DTT (Supplementary Fig. S1). Under DTT treatment, the amounts of spliced HXL1 mRNA in the cna1 and cnb1 gene-deficient mutants were not altered compared to the parent strain (Supplementary Fig. S1). These results suggest that DTT induces the HXL1 mRNA splicing independent to calcineurin signaling in T. asahii.
Role of calcineurin in hyphal formation by T. asahii
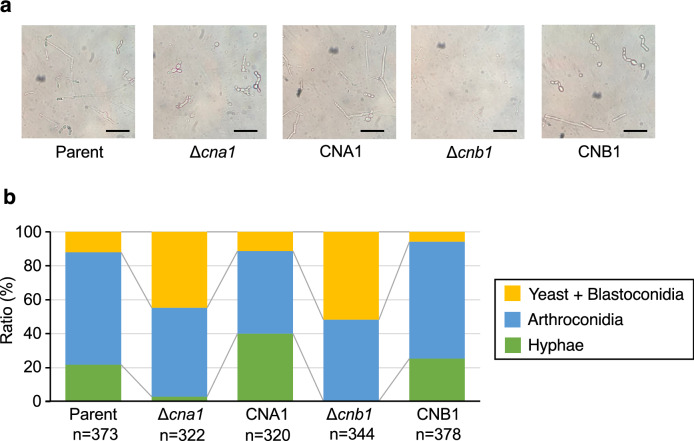
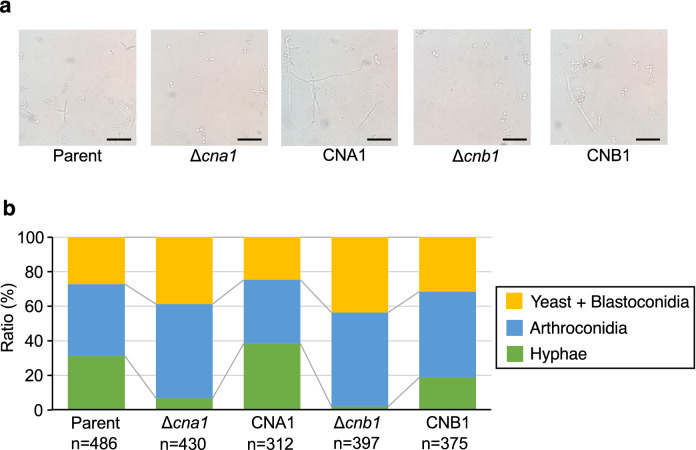
Trichosporon asahii has several morphologic forms: yeast, hyphae (filament form), and arthroconidia (chains of cells and asexual spores)4. In Candida tropicalis, calcineurin is a key factor that regulates to form hyphae33. We examined whether cna1 and cnb1 gene deficiencies affect hyphal formation in T. asahii. In Sabouraud dextrose medium, the hyphae ratio was lower in the cna1 and cnb1 gene-deficient mutants than in the parental strain (Fig. 4). The phenotype of lower hyphal forming activities in these gene-deficient mutants was suppressed in their revertants (Fig. 4). These observations suggest that the cna1 and cnb1 genes contribute to hyphal formation by T. asahii.
Figure 4.
Effect of cna1 or the cnb1 gene-deficiency on T. asahii morphology in Sabouraud dextrose medium. The T. asahii parent strain (Parent), the cna1 gene-deficient mutant (∆cna1), the revertant of ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant of ∆cnb1 (CNB1) were grown on SDA and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution and filtered through a 40-μm cell strainer. Absorbance at 630 nm of the T. asahii cell suspension was adjusted to 1–1.5. The cell suspension (10 µl) was added to Sabouraud dextrose medium (100 µl). The solutions were incubated at 37 °C for 48 h. The incubated solution was placed on glass slides and covered by a glass coverslip. (a) Samples were examined with bright light under a microscope. The scale bar indicated 50 µm. (b) The pictures were randomly obtained. The numbers of three cell types: yeast + blastoconidia, arthroconidia, and hyphae, were counted. The representative cells to determine the cell types are shown in Supplementary Fig. S2.
Calcineurin-dependency of T. asahii virulence against silkworms
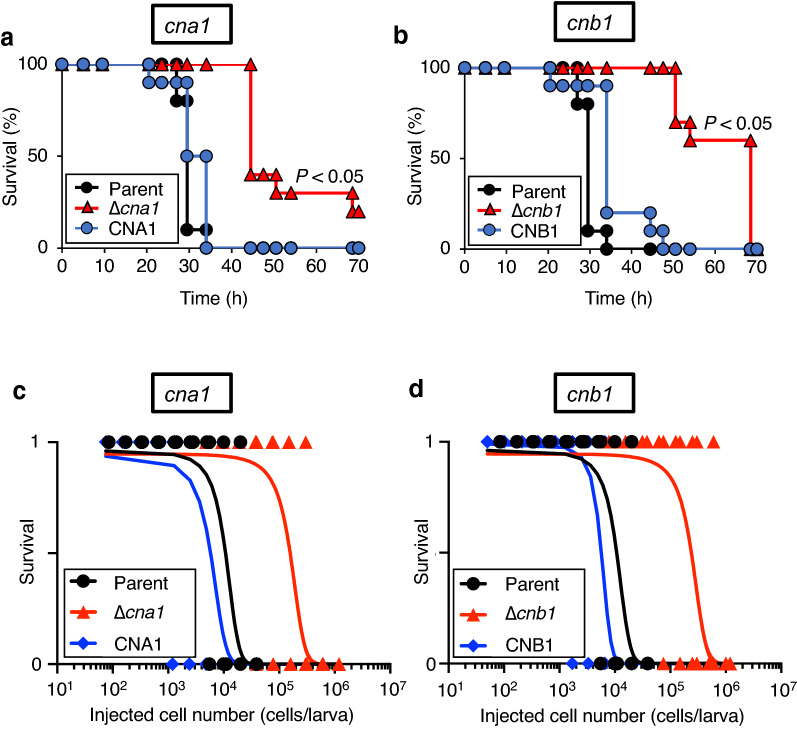
In C. neoformans, the cna1 gene is required for virulence against silkworms34. We examined whether deficiencies of the cna1 and cnb1 genes reduced the virulence of T. asahii against silkworms. Survival times of silkworms injected with the cna1 and cnb1 gene-deficient mutants were longer than those of the parental strain (Fig. 5a,b). The half-maximal lethal dose (LD50) values of the cna1 and cnb1 gene-deficient mutants was more than tenfold higher that of the parent strain (Fig. 5c,d, and Table 1). These phenotypes were suppressed in their revertants (Fig. 5 and Table 1). These findings suggest that the cna1 and cnb1 genes are involved in the virulence of T. asahii against silkworms.
Figure 5.
Attenuated pathogenicity in the cna1 or the cnb1 gene-deficient T. asahii mutants against silkworms. (a,b) The T. asahii parent strain (Parent; 2.9 × 105 cells/larva), the cna1 gene-deficient mutant (∆cna1; 7.4 × 105 cells/larva), the revertant from ∆cna1 (CNA1; 4.2 × 105 cells/larva), the cnb1 gene-deficient mutant (∆cnb1; 6.1 × 105 cells/larva), or the revertant from ∆cnb1 (CNB1; 7.1 × 105 cells/larva) were injected into the silkworm hemolymph and the silkworms were incubated at 37 °C. Silkworm survival was monitored for 72 h. The significance of differences between the parent strain group and the cnb1 gene-deficient mutant groups was calculated by the log-rank test based on the curves by the Kaplan–Meier method. P < 0.05 was considered significant. n = 10/group. (c,d) Number of surviving silkworms at conditions under 37 °C was determined at 48 h after administration of the fungal cells (50 to 1.2 × 106 cells/larva) into the silkworm hemolymph. Surviving and dead silkworms are indicated as 1 and 0, respectively. n = 4/group. Curves were drawn from combined data of 2–3 independent experiments by a simple logistic regression model.
Table 1.
The LD50 values of T. asahii strains.
| T. asahii strains | LD50 (× 103 cells/larva) |
|---|---|
| Parent strain | 11 |
| ∆cna1 | 160 |
| CNA1 (Revertant) | 6 |
| ∆cnb1 | 240 |
| CNB1 (Revertant) | 6 |
Calcineurin-dependency of T. asahii morphological change in silkworm hemolymph and human serum
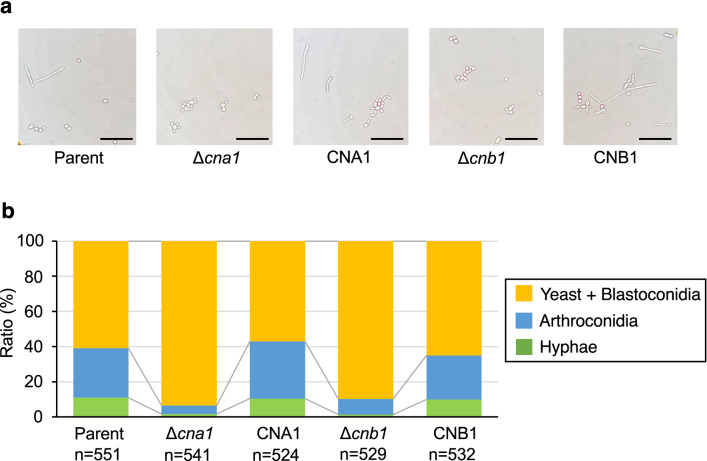
We examined the effects of deficiencies of the cna1 and cnb1 genes on T. asahii morphology in the host environments. In the silkworm hemolymph, the hyphal ratio in the cna1 and cnb1 gene-deficient mutants was lower than that of the parent strain (Fig. 6). In human serum, the hyphae and arthroconidia ratio was also lower in the cna1 and cnb1 gene-deficient mutants than in the parental strain (Fig. 7). These phenotypes were suppressed in their revertants (Figs. 6 and 7). The results suggest that the cna1 and cnb1 genes are involved in the T. asahii morphological change in the host environments.
Figure 6.
Low hyphal growth of cna1 or the cnb1 gene-deficient T. asahii mutants in harvested silkworm hemolymph. The T. asahii parent strain (Parent), the cna1 gene-deficient mutant (∆cna1), the revertant of ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant of ∆cnb1 (CNB1) were grown on SDA and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution and filtered through a 40-μm cell strainer. Absorbance at 630 nm of the T. asahii cell suspension was adjusted to 1–1.5. The cell suspension (10 µl) was added to harvested silkworm hemolymph (100 µl) and the solutions were incubated at 37 °C for 48 h. The incubated solution was placed on glass slides and covered by a glass coverslip. (a) Samples were examined with bright light under a microscope. The scale bar indicated 50 µm. (b) The pictures were randomly obtained. The numbers of three cell types: yeast + blastoconidia, arthroconidia, and hyphae, were counted.
Figure 7.
Effect of cna1 or the cnb1 gene-deficiency on T. asahii morphology in human serum. The T. asahii parent strain (Parent), the cna1 gene-deficient mutant (∆cna1), the revertant of ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant of ∆cnb1 (CNB1) were grown on SDA and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution and filtered through a 40-μm cell strainer. Absorbance at 630 nm of the T. asahii cell suspension was adjusted to 1–1.5. The cell suspension (10 µl) was added to human serum (100 µl) and the solutions were incubated at 37 °C for 48 h. The incubated solution was placed on glass slides and covered by a glass coverslip. (a) Samples were examined with bright light under a microscope. The scale bar indicated 50 µm. (b) The pictures were randomly obtained. The numbers of three cell types: yeast + blastoconidia, arthroconidia, and hyphae, were counted.
Discussion
The present study using cna1 and cnb1 gene-deficient mutants demonstrated that calcineurin in T. asahii plays a vital role in stress resistance and virulence against silkworms. Our findings suggest that the pathogenicity of T. asahii is regulated via the calcineurin-signaling pathway.
The calcineurin-signaling pathway is required for normal growth of T. asahii under a high-temperature condition (40 °C). Severe growth inhibition of the cna1 and cnb1 gene-deficient T. asahii mutants were observed at 40 °C compared with those at 27 °C and 37 °C. In C. neoformans, calcineurin is required for the growth at 37 °C29,30. Therefore, the high-temperature stress response is partially regulated by calcineurin in T. asahii compared with C. neoformans.
In Cryptococcus gattii and C. neoformans, calcineurin plays an important role in cell membrane homeostasis, cell wall integrity, the ER stress response, cation homeostasis, and fluconazole tolerance21,22. Calcineurin in T. asahii is related to resistance against cell membrane and cell wall damage, and ER stress, but not cell wall integrity dependent on the CWI pathway, osmotic stress resistance, cation homeostasis, or antifungal drug resistance. The cna1 gene-deficient mutant of C. neoformans exhibits sensitivities to SDS, Congo red, caffeine, sorbitol, BFA, monensin, DTT, TM, CaCl2, LiCl, NaCl, and fluconazole21,22,35,36. Therefore, the roles of calcineurin in cell wall integrity dependent on the CWI pathway, osmotic stress resistance, cation homeostasis, and antifungal drug resistance differ between T. asahii and C. neoformans. Cheon and colleagues reported that DTT induces the Ire1-mediated UPR signaling in C. neoformans31. In T. asahii, DTT also induced the HXL1 mRNA splicing by Ire1 protein. The HXL1 mRNA splicing induced by DTT occurred in the cna1 and cnb1 gene-deficient T. asahii mutants. Therefore, calcineurin does not regulate Ire1-mediated UPR signaling caused by DTT in T. asahii, at least in the experimental condition. Revealing the molecular mechanisms of calcineurin on the stress response in T. asahii is an important future subject.
Trichosporon asahii forms hyphae in Sabouraud dextrose medium and in silkworm hemolymph via calcineurin. Moreover, calcineurin is required for T. asahii virulence in a silkworm infection model. In Candida dubliniensis, Candida tropicalis, and Aspergillus fumigatus, the calcineurin signaling pathway regulates hyphal growth and virulence37,38. T. asahii forms hyphae in the hemolymph of silkworms infected with T. asahii23. Hyphal growth of T. asahii in blood vessels causes necrotic thrombi and may contribute to infection39. Moreover, we demonstrated that the calcineurin contributes to arthroconidia and hyphal formation of T. asahii in human serum. Therefore, morphological change of T. asahii regulated by calcineurin might contribute to its virulence against silkworms and humans. Further studies are needed to reveal the role of the calcineurin in T. asahii virulence using a mouse infection model. The calcineurin in C. neoformans regulates gene expression via dephosphorylation of the transcription factors40. We speculate that the calcineurin in T. asahii regulates hyphal formation-related gene expression. Identifying the crucial genes involved in hyphal formation regulated by calcineurin in T. asahii will be an important next step.
Tacrolimus (FK506), a calcineurin inhibitor, affects the physiologies of several pathogenic fungi41,42. On the other hand, FK506 suppresses the immune system by blocking T-cell activation in human43. Therefore, the immunosuppressive effect should be eliminated when used as a treatment for fungal infections. Juvvadi and colleagues succeeded to develop the low immunosuppressive FK506 analog APX879 that inhibits the Aspergillus fumigatus calcineurin44. We assumed that the fungal calcineurin specific inhibitors might be effective against T. asahii infection.
In conclusion, calcineurin in T. asahii has roles in the stress response, hyphal formation, and virulence. We assumed that calcineurin is a potent target for anti-infectious agents against T. asahii infection.
Methods
Reagents
SDS, sorbitol, DTT, NaCl, and LiCl were purchased from Wako Pure Chemical Industries (Osaka, Japan). Fluconazole, voriconazole, hygromycin B, caffeine, and monensin sodium salt were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Nourseothricin was purchased from Jena Bioscience (Dortmund, Germany). Congo red was purchased from Sigma-Aldrich (St. Louis, MO, USA). G418 was purchased from Enzo Life Science, Inc. (Farmingdale, NY, USA). CaCl2 was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Tunicamycin was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Brefeldin A (BFA) was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Human serum [(from male AB clotted whole blood), USA origin, sterile-filtered] (Product ID: H6914) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Culture of T. asahii
The T. asahii strain (MPU129 ku70 gene-deficient mutant) used in this study was generated as previously reported28. The T. asahii MPU129 ku70 gene-deficient mutant was grown on Sabouraud dextrose agar (SDA) (1% hipolypepton [Nihon Pharmaceutical Co., Ltd., Tokyo, Japan], 4% dextrose, and 1.5% agar [both from FUJIFILM Wako Pure Chemical Industries, Osaka, Japan]) containing G418 (50 μg/ml) and incubated at 27 °C for 2 days.
Construction of gene-deficient T. asahii mutants and their revertants
The plasmid for gene-deficient T. asahii strains was constructed according to a previous report45. To generate the cna1 or cnb1 gene-deficient strains, the 5’-UTR and 3’-UTR of the cna1 or cnb1 gene were introduced into a pAg1-NAT1 vector46. To generate their revertants, the hygromycin phosphotransferase gene (hph) cassette and the cna1 or cnb1 gene were introduced into pAg1-cna1(5’UTR)-NAT1-cna1(3’UTR) or pAg1-cnb1(5’UTR)-NAT1-cnb1(3’UTR). Cloning was performed by the infusion method (In-Fusion HD Cloning Kit, Takara, Shiga, Japan) or ligation method (DNA Ligation Kit Ver.2.1, Takara, Shiga, Japan). The primers used for PCR amplification of each DNA region are shown in Supplementary Table S1.
Competent cells for electroporation were prepared according to a previous report28. T. asahii was spread on an SDA plate and cultured at 27 °C for 1 day. T. asahii cells on agar were suspended by physiologic saline solution (2 ml), and the suspension was transferred to a 1.5-ml tube. The fungal cells were collected by centrifugation at 8000 rpm for 3 min (TOMY-MX100, TOMY Digital Biology Co. Ltd, Tokyo, Japan) and suspended by adding 1 ml of ice-cold water and centrifuged at 8000 rpm for 3 min. This washing process was repeated 4 times. The washed cells were suspended by adding 1 ml of 1.2 M sorbitol solution and centrifuged at 8000 rpm for 3 min. The obtained fungal cells were suspended with 0.2 ml of 1.2 M sorbitol solution as competent cells. The PCR-amplified 5'-UTR (cna1) -NAT1-3'-UTR (cna1), 5'-UTR (cna1) -cna1-hph-3'-UTR (cna1), 5'-UTR (cnb1) -NAT1-3'-UTR (cnb1), or 5'-UTR (cnb1) -cnb1-hph-3'-UTR (cnb1) fragments (180 ng/2 µl) were added to the T. asahii competent cells (40 µl) and placed on ice for 15 min. The suspension was added to a 0.2-cm gap cuvette (Bio-Rad Laboratories, Inc.) and electroporated (Time constant protocol: 1800 V, 5 ms) using a Gene Pulser Xcell (Bio-Rad Laboratories, Inc.). The cells were suspended by adding 500 µl YPD containing 0.6 M sorbitol and incubated at 27 °C for 3 h. After incubation, the cells were collected by centrifugation at 10,000 rpm for 5 min and suspended in 100 µl of physiologic saline solution and applied to SDA containing nourseothricin (300 µg/ml) or hygromycin B (300 µg/ml). The cells were incubated at 27 °C for 3 days and the growing colonies were isolated as gene-deficient strain or revertant candidates. Introduction of the mutation into the genome of the candidate strains was confirmed using the extracted genome as a template DNA by PCR with the primers shown in Supplementary Table S1. The information of strains were shown in Table 2.
Table 2.
T. asahii strains used in this study.
| T. asahii strains | Relevant genotype | Background | Reference |
|---|---|---|---|
| MPU129 ∆ku70 (Parent strain) | ku70::nptII | MPU129 | Matsumoto et al.28 |
| ∆cna1 | ku70::nptII, cna1::NAT1 | MPU129 ∆ku70 | This study |
| CNA1 (Revertant) | ku70::nptII, NAT1::cna1, hgh | MPU129 ∆ku70 ∆cna1 | This study |
| ∆cnb1 | ku70::nptII, cnb1::NAT1 | MPU129 ∆ku70 | This study |
| CNB1 (Revertant) | ku70::nptII, NAT1::cnb1, hgh | MPU129 ∆ku70 ∆cnb1 | This study |
Temperature sensitivity test
The T. asahii strains were grown on SDA containing G418 (50 μg/ml) and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution (0.9% w/v NaCl) and filtered through a 40-μm cell strainer (Corning Inc., Corning, NY, USA). Absorbance of 630 nm of the T. asahii cell suspension was adjusted to 1. Series of tenfold dilution of the fungal suspension were prepared using saline. Five microliters of each cell suspension was spotted on the SDA. The agar plates were incubated at 27, 37, or 40 °C for 24 h, and photographs were obtained.
For growth on liquid medium, Sabouraud liquid medium (1% hipolypepton, 4% dextrose) was used in this study. Suspensions of the T. asahii parent strain (Parent) the cna1 gene-deficient mutant (∆cna1), the revertant from ∆cna1 (CNA1), the cnb1 gene-deficient mutant (∆cnb1), and the revertant from ∆cnb1 (CNB1) were prepared with Sabouraud medium and adjusted to 0.005 on absorbance at 630 nm. The T. asahii suspensions were incubated at 27 °C, 37 °C, or 40 °C for 4 days and absorbance at 630 nm was measured using a microplate reader (iMark™ microplate reader; Bio-Rad Laboratories Inc., Hercules, CA, USA).
Drug sensitivity test
The T. asahii strains were grown on SDA containing G418 (50 μg/ml) and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution (0.9% w/v NaCl) and filtered through a 40-μm cell strainer (Corning Inc.). Absorbance of 630 nm of the T. asahii cell suspension was adjusted to 1. Series of tenfold dilution of the fungal suspension were prepared using saline. Five microliters of each cell suspension was spotted on the SDA containing SDS (0.00625%), Congo red (100 µg/ml), caffeine (0.65 mg/ml), sorbitol (1.5 M), DTT (12 mM), TM (1 µg/ml), BFA (10 µg/ml), Monensin (1 mg/ml), CaCl2 (0.4 M), LiCl (55 mM), NaCl (1 M), amphotericin B (0.4 µg/ml), fluconazole (6.4 µg/ml), or voriconazole (0.12 µg/ml). Each agar plate was incubated at 37 °C for 24 h, and photographs were obtained.
Observation of T. asahii morphology
The T. asahii strains were grown on SDA containing G418 (50 μg/ml) and incubated at 27 °C for 2 days. T. asahii cells was suspended in physiologic saline solution and filtered through a 40-μm cell strainer (Corning Inc.). Absorbance of 630 nm of the T. asahii cell suspension was adjusted to 1–1.5. The cell suspension (10 µl) was added to Sabouraud dextrose medium, harvested silkworm hemolymph, or human serum (100 µl). The solutions were incubated at 37 °C for 48 h. The incubated solution was placed on glass slides and covered by a glass coverslip. Samples were examined with bright light under a microscope (CH-30; Olympus, Tokyo, Japan). The pictures were randomly obtained. The morphology of T. asahii was determined according to a previous report15. The numbers of the 3 types of cells, yeast, arthroconidia, and hyphae, were counted.
Silkworm infection experiments
Silkworm infection experiments were performed according to a previous report23. Eggs of silkworms (Hu Yo × Tukuba Ne) were purchased from Ehime-Sanshu Co, Ltd. (Ehime, Japan), disinfected, and hatched at 25–27 °C. The silkworms were fed an artificial diet, Silkmate 2S, containing antibiotics purchased from Ehime-Sanshu Co., Ltd. Fifth instar larvae were used in the infection experiments. Silkworm fifth instar larvae were fed the artificial diet (Silkmate 2S; Ehime-Sanshu Co., Ltd.) overnight. T. asahii grown on SDA plates was suspended in physiologic saline solution (0.9% w/v NaCl) and filtered through a 40-μm cell strainer (Corning Inc.). A 50-µl suspension of T. asahii cells was administered to the silkworm hemolymph by injecting the silkworm dorsally using a 1-ml tuberculin syringe (Terumo Medical Corporation, Tokyo, Japan). Silkworms injected with T. asahii cells were placed in an incubator and their survival was monitored.
LD50 measurement
The dose of T. asahii required to kill half of the silkworms (LD50) was determined according to the previous report23. T. asahii strains (1 × 102 to 2 × 107 cells/50 µl) were injected into the silkworm hemolymph and the silkworms were incubated at 37 °C. Survival of the silkworms (n = 4/group) at 48 h was recorded. The LD50 was determined from the combined data of 2–3 independent experiments by a simple logistic regression model using Prism 9.1.2 (GraphPad Software, LLC, San Diego, CA, USA, https://www.graph pad.com/scientific -software/prism/).
Statistical analysis
All experiments were performed at least twice and representative results are shown. The significance of differences between groups in the silkworm infection experiments was calculated by the log-rank test based on curves determined by the Kaplan–Meier method using Prism 9.1.2. P < 0.05 was considered significant.
Supplementary Information
Acknowledgements
We thank Eri Sato, Hiromi Kanai, and Sachi Koganesawa (Meiji Pharmaceutical University) for their technical assistance rearing the silkworms. This study was supported by JSPS KAKENHI grant number JP20K07022 (Scientific Research (C) to Y.M.) and in part by the Research Program on Emerging and Re-emerging Infectious Diseases of the Japan Agency for Medical Research and Development, AMED (Grant number JP20fk0108135h0201 to T.S.).
Author contributions
Study conception and design: Y.M. Acquisition of data: Y.M., A.Y., T.N., Y.S., T.Y. Analysis and interpretation of data: Y.M., A.Y., T.N., Y.S. Drafting of manuscript: Y.M. Critical revision: Y.M., A.Y., T.N., T.Y., T.S. All authors have read and approved the final version of the manuscript.
Data availability
The datasets generated during the present study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20507-x.
References
- 1.Sugita T, Nishikawa A, Ichikawa T, Ikeda R, Shinoda T. Isolation of Trichosporon asahii from environmental materials. Med. Mycol. 2000;38:27–30. doi: 10.1080/mmy.38.1.27.30. [DOI] [PubMed] [Google Scholar]
- 2.Sugita T, et al. Genetic diversity and biochemical characteristics of Trichosporon asahii isolated from clinical specimens, houses of patients with summer-type-hypersensitivity pneumonitis, and environmental materials. J. Clin. Microbiol. 2001;39:2405–2411. doi: 10.1128/JCM.39.7.2405-2411.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang E, Sugita T, Tsuboi R, Yamazaki T, Makimura K. The opportunistic yeast pathogen Trichosporon asahii colonizes the skin of healthy individuals: Analysis of 380 healthy individuals by age and gender using a nested polymerase chain reaction assay. Microbiol. Immunol. 2011;55:483–488. doi: 10.1111/j.1348-0421.2011.00341.x. [DOI] [PubMed] [Google Scholar]
- 4.Colombo AL, Padovan ACB, Chaves GM. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin. Microbiol. Rev. 2011;24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouba N, Raoult D, Drancourt M. Eukaryote culturomics of the gut reveals new species. PLoS ONE. 2014;9:e106994. doi: 10.1371/journal.pone.0106994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho O, Matsukura M, Sugita T. Molecular evidence that the opportunistic fungal pathogen Trichosporon asahii is part of the normal fungal microbiota of the human gut based on rRNA genotyping. Int. J. Infect. Dis. 2015;39:87–88. doi: 10.1016/j.ijid.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TJ, et al. Experimental Trichosporon infection in persistently granulocytopenic rabbits: Implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J. Infect. Dis. 1992;166:121–133. doi: 10.1093/infdis/166.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Walsh TJ, Melcher GP, Lee JW, Pizzo PA. Infections due to Trichosporon species: New concepts in mycology, pathogenesis, diagnosis and treatment. Curr. Top. Med. Mycol. 1993;5:79–113. [PubMed] [Google Scholar]
- 9.Duarte-Oliveira C, et al. The cell biology of the Trichosporon-host interaction. Front. Cell Infect. Microbiol. 2017;7:118. doi: 10.3389/fcimb.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krcmery V, et al. Hematogenous trichosporonosis in cancer patients: Report of 12 cases including 5 during prophylaxis with itraconazol. Support Care Cancer. 1999;7:39–43. doi: 10.1007/s005200050221. [DOI] [PubMed] [Google Scholar]
- 11.Kimura M, et al. Micafungin breakthrough fungemia in patients with hematological disorders. Antimicrob. Agents Chemother. 2018;62:324. doi: 10.1128/AAC.02183-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toriumi Y, Sugita T, Nakajima M, Matsushima T, Shinoda T. Antifungal pharmacodynamic characteristics of amphotericin B against Trichosporon asahii, using time-kill methodology. Microbiol. Immunol. 2002;46:89–93. doi: 10.1111/j.1348-0421.2002.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 13.Iturrieta-González IA, Padovan ACB, Bizerra FC, Hahn RC, Colombo AL. Multiple species of Trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS ONE. 2014;9:e109553. doi: 10.1371/journal.pone.0109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Bonaventura G, et al. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: Development, architecture, and antifungal resistance. Antimicrob. Agents Chemother. 2006;50:3269–3276. doi: 10.1128/AAC.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurakado S, et al. Role of arthroconidia in biofilm formation by Trichosporon asahii. Mycoses. 2021;64:42–47. doi: 10.1111/myc.13181. [DOI] [PubMed] [Google Scholar]
- 16.Hemenway CS, Heitman J. Calcineurin. Structure, function, and inhibition. Cell Biochem. Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 17.Kozubowski L, Lee SC, Heitman J. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 2009;11:370–380. doi: 10.1111/j.1462-5822.2008.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer KR, Robbins N, Cowen LE. The role of Candida albicans stress response pathways in antifungal tolerance and resistance. iScience. 2022;25:103953. doi: 10.1016/j.isci.2022.103953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odom A, et al. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutherford JC, Bahn Y-S, van den Berg B, Heitman J, Xue C. Nutrient and stress sensing in pathogenic yeasts. Front. Microbiol. 2019;10:442. doi: 10.3389/fmicb.2019.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y-L, Lehman VN, Lewit Y, Averette AF, Heitman J. Calcineurin governs thermotolerance and virulence of Cryptococcus gattii. G3. 2013;3:527–539. doi: 10.1534/g3.112.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu G, et al. The monothiol glutaredoxin Grx4 influences thermotolerance, cell wall integrity, and Mpk1 signaling in Cryptococcus neoformans. G3. 2021;11:322. doi: 10.1093/g3journal/jkab322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto Y, et al. A novel silkworm infection model with fluorescence imaging using transgenic Trichosporon asahii expressing eGFP. Sci. Rep. 2020;10:10991–11011. doi: 10.1038/s41598-020-67841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto Y. Facilitating drug discovery in human disease models using insects. Biol. Pharm. Bull. 2020;43:216–220. doi: 10.1248/bpb.b19-00834. [DOI] [PubMed] [Google Scholar]
- 25.Flecknell P. Replacement, reduction and refinement. Altex. 2002;19:73–78. [PubMed] [Google Scholar]
- 26.Gokaslan A, Anaissie E. A novel murine model of disseminated trichosporonosis. Infect. Immun. 1992;60:3339–3344. doi: 10.1128/iai.60.8.3339-3344.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montoya AM, et al. In vivo pathogenicity of Trichosporon asahii isolates with different in vitro enzymatic profiles in an immunocompetent murine model of systemic trichosporonosis. Med. Mycol. 2018;56:434–441. doi: 10.1093/mmy/myx057. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto Y, et al. Development of an efficient gene-targeting system for elucidating infection mechanisms of the fungal pathogen Trichosporon asahii. Sci. Rep. 2021;11:18270–18310. doi: 10.1038/s41598-021-97287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz MC, Sia RA, Olson M, Cox GM, Heitman J. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 2000;68:982–985. doi: 10.1128/IAI.68.2.982-985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox DS, et al. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 2001;39:835–849. doi: 10.1046/j.1365-2958.2001.02295.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheon SA, et al. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 2011;7:e1002177. doi: 10.1371/journal.ppat.1002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheon SA, Jung K-W, Bahn Y-S, Kang HA. The unfolded protein response (UPR) pathway in Cryptococcus. Virulence. 2014;5:341–350. doi: 10.4161/viru.26774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y-L, et al. Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryot. Cell. 2014;13:844–854. doi: 10.1128/EC.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto Y, et al. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 2012;112:138–146. doi: 10.1111/j.1365-2672.2011.05186.x. [DOI] [PubMed] [Google Scholar]
- 35.Park H-S, et al. Calcineurin targets involved in stress survival and fungal virulence. PLoS Pathog. 2016;12:e1005873. doi: 10.1371/journal.ppat.1005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu C, Donadio N, Cardenas ME, Heitman J. Dissecting the roles of the calcineurin pathway in unisexual reproduction, stress responses, and virulence in Cryptococcus deneoformans. Genetics. 2018;208:639–653. doi: 10.1534/genetics.117.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y-L, et al. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot. Cell. 2011;10:803–819. doi: 10.1128/EC.00310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juvvadi PR, Lamoth F, Steinbach WJ. Calcineurin-mediated regulation of hyphal growth, septation, and virulence in Aspergillus fumigatus. Mycopathologia. 2014;178:341–348. doi: 10.1007/s11046-014-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mada PK, Ayoade F, Li A, Todd J. Trichosporon asahii septic thrombophlebitis following lower extremity amputation in an immunocompetent host. BMJ Case Rep. 2018;2018:221441. doi: 10.1136/bcr-2017-221441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto Y, et al. Induction of signal transduction pathways related to the pathogenicity of Cryptococcus neoformans in the host environment. Drug Discov. Ther. 2019;13:177–182. doi: 10.5582/ddt.2019.01047. [DOI] [PubMed] [Google Scholar]
- 41.Steinbach WJ, Reedy JL, Cramer RA, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007;5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 42.Yu S-J, Chang Y-L, Chen Y-L. Calcineurin signaling: Lessons from Candida species. FEMS Yeast Res. 2015;15:fov016. doi: 10.1093/femsyr/fov016. [DOI] [PubMed] [Google Scholar]
- 43.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can't live without…. J. Immunol. 2013;191:5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 44.Juvvadi PR, et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat. Commun. 2019;10:4275–4318. doi: 10.1038/s41467-019-12199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada T, et al. Enhanced gene replacements in Ku80 disruption mutants of the dermatophyte, Trichophyton mentagrophytes. FEMS Microbiol. Lett. 2009;298:208–217. doi: 10.1111/j.1574-6968.2009.01714.x. [DOI] [PubMed] [Google Scholar]
- 46.Alshahni MM, Makimura K, Yamada T, Takatori K, Sawada T. Nourseothricin acetyltransferase: A new dominant selectable marker for the dermatophyte Trichophyton mentagrophytes. Med. Mycol. 2010;48:665–668. doi: 10.3109/13693780903330555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the present study are available from the corresponding author on reasonable request.