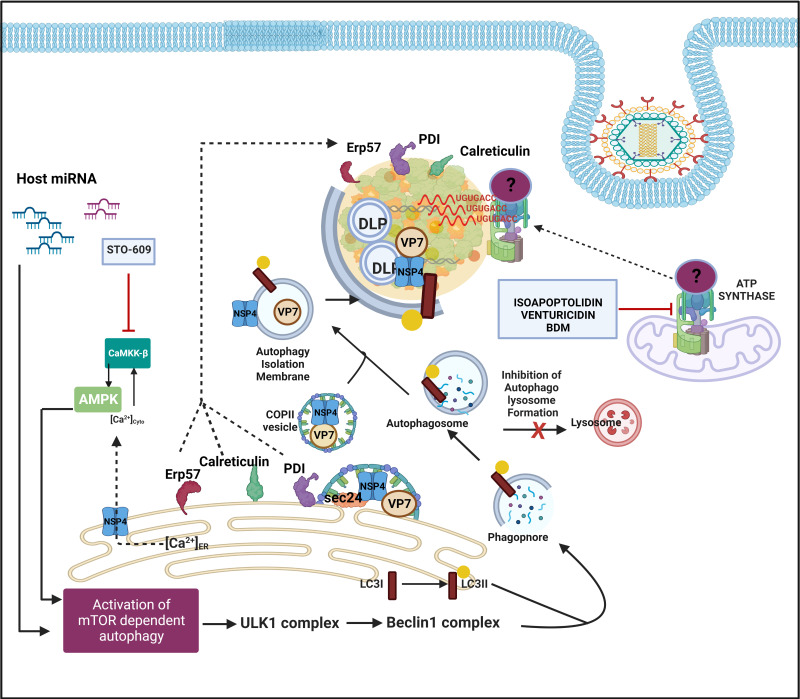
Figure 5.
Host cellular contribution in the later events of viroplasm dynamics. Many ER chaperone proteins such as Erp57, PDI, and Calreticulin translocate near RV viroplasms. PDI and Calreticulin foster RV morphogenesis. In RV infected cells, mTOR inhibition and the autophagic signalling are induced by host microRNA-dependent mechanism and also via NSP4-Ca2+-Calmodulin-CaMKKβ-AMPK pathway. Overall, mTOR restriction causes de-repression of the ULK1 complex which subsequently forms phagophore through Beclin1 complex activation and LC3 II lipidation. However, autophagosomes are prevented from lysosomal targeting in RV infected cells; instead, they are utilized for carrying the RV proteins NSP4 and VP7 coming out with the ER-derived COP-II vesicles to maturing progeny virions within viroplasms, thereby aiding in outer capsid assembly. Inhibiting CaMKKβ by STO-609 abrogates the presence of NSP4 and VP7 to reside surrounding autophagosome-engulfed viroplasms, leading to curtailed RV progeny yield. Other host contributors which aid in the RV morphogenesis within viroplasms are subunits (ATP5B, ATP5A1, ATP5O) of the mitochondrial ATP synthase holoenzyme that re-locate to viroplasms and interact with the 3′ UTR consensus of RV RNAs (5′-UGUGACC-3′). Potential involvement of an intermediate protein has been speculated to facilitate the ATP synthase-RV RNA 3′ UTR association. Chemical inhibitors targeting ATP synthase such as Isoapoptolidin, Venturicidin, and BDM antagonize RV progeny yield.