FIGURE 7.
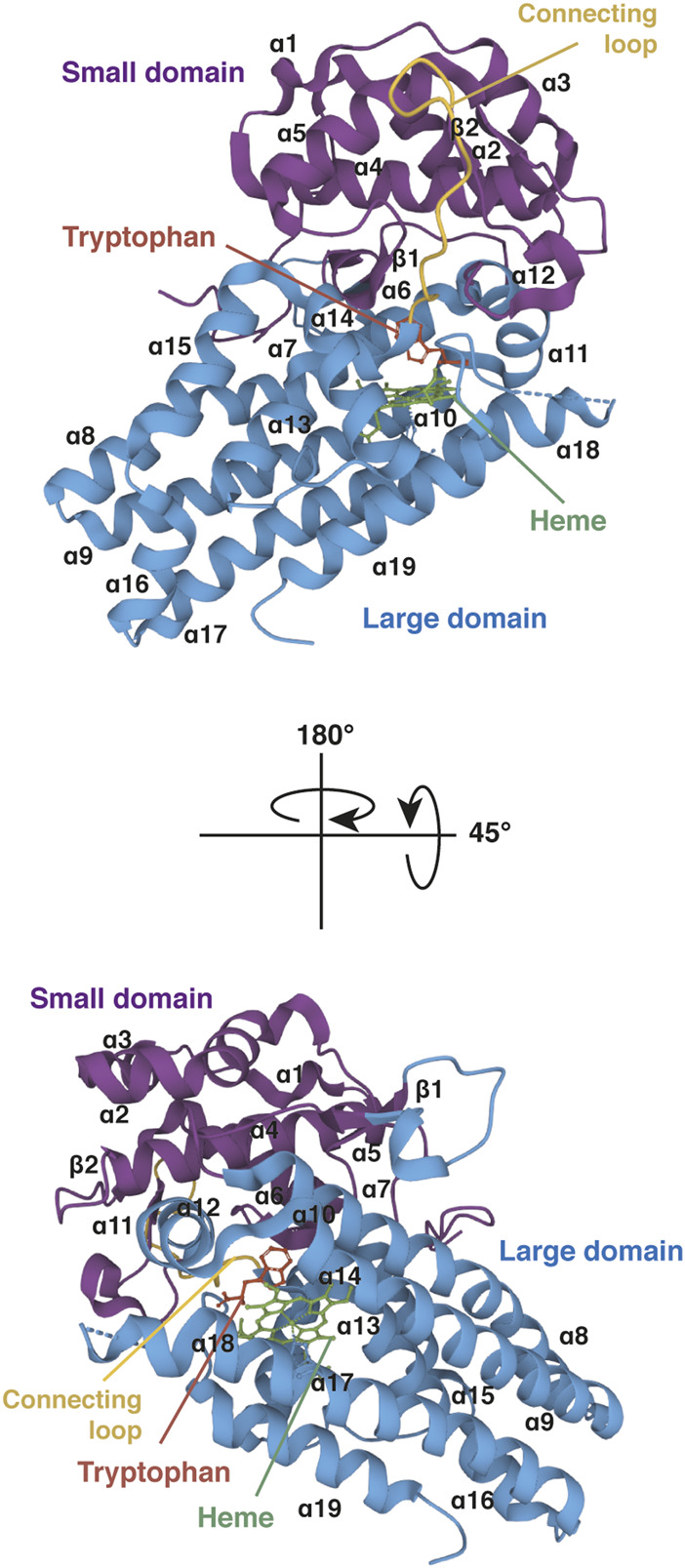
Crystal structure of human IDO1 (holo protein). Structure of monomeric IDO1 complexed with tryptophan (red) and the heme cofactor (green) in two orientations. The secondary structure of IDO1 comprises nineteen α-helices and two β-strands assembled in a large domain (blue) and a small domain (purple) arranged on either side of the heme binding site. Both domains are connected together by a long loop (yellow). The heme binding site is surrounded at the proximal side by α-helices 7, 9, 17, and 19 of the large domain and at the distal side by the small domain and the connecting loop. The proximal side refers to the proximal histidine forming a coordinate covalent bound with the iron ion at the center of the heme group, and the distal side to the other side of the heme where iron binds dioxygen. The tryptophan-binding site is shaped by α-helices 11, 12, and 14 of the large domain and the connecting loop, close to the distal side of the heme. The 3D structure of IDO1 was modelized with Mol* Viewer from the crystal structure of IDO1 described by Luo et al. (2018) (PDB file ID: 6E46).
