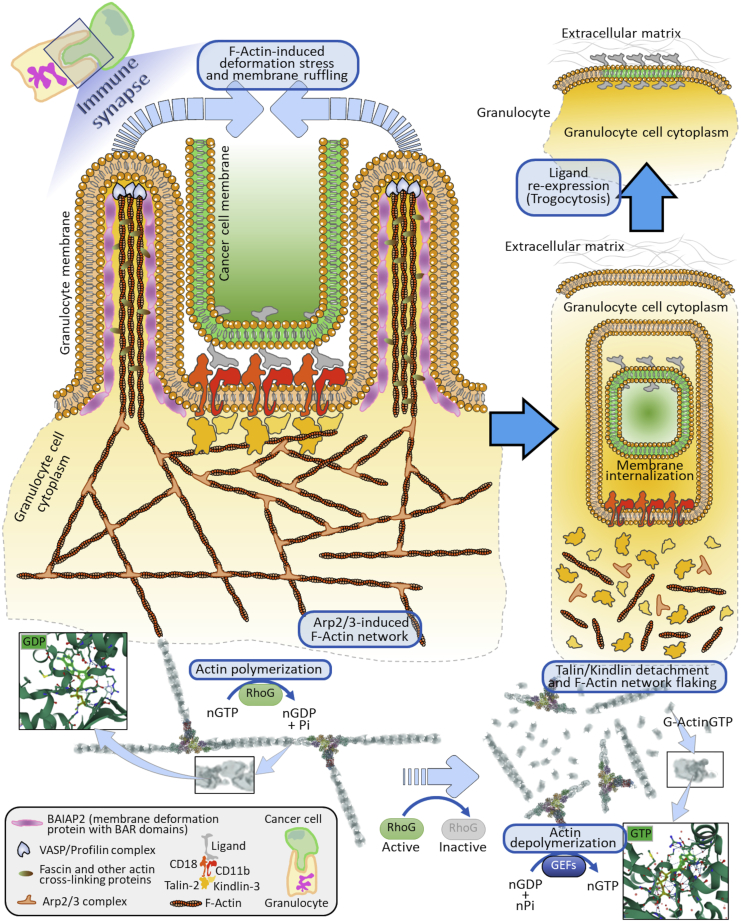
Figure 9.
Hypothetic molecular model for the role of F-actin network reorganization as a main biomechanical driver for granulocyte trogocytosis of cancer cells
The generation and maintenance of an immune synapse starts with CD18/CD11b integrins-ligand complex clusterization inside the granulocyte membrane surface (orange-color membrane). These clusters elicit the recruitment of talin-2 and kindlin-3, which bind to the cytosolic regions of integrins. Talin-2 and kindlin-3 also associate with the F-actin fibers, thus activating the accumulation of F-actin filaments tight to the clusters. Through the Arp2/3 complexes, the F-actin network extends locally and at the sides of the clusters leading to the formation of F-actin fibers. VASP, Fascins, and BAIAP2 elicit the generation of peculiar tubular membrane structures, whose formation and stability are sustained by actin polymerization via active RhoG, which also leads to the local extension of the actin network by the degradation of GTP into GDP and phosphate (Pi). GDP then interacts with actin monomers (upper left box). At this point, these tubular architectures, guided by the actin network diffusion force, will entrap the cancer cell membrane (green color) and ligands. In parallel, the ligands on the cancer cell membrane detach from the integrins located on the granulocyte membrane. This leads to the spontaneous dissociation of talin-2 and kindlin-3 from integrins and in turn to F-actin decomplexation, with subsequent actin network disaggregation. Concomitantly, the prevalence of inactive RhoG stimulates the GEFs-dependent F-Actin depolymerization and GEFs-guided GTP formation (lower right box), culminating with the exposition of ligand molecules into the granulocyte membrane by sequential membrane fusion events, thus terminating the process of trogocytosis.