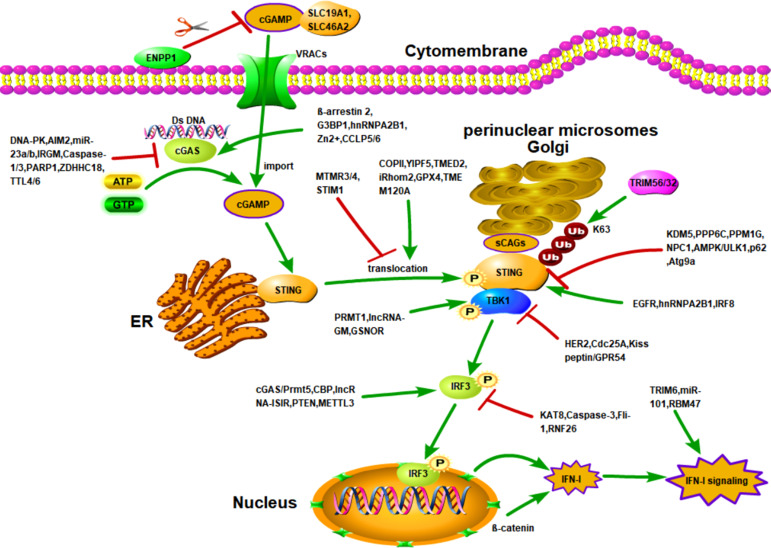
Figure 1.
Activation of cGAS/STING signaling and regulation of cGAS/STING signaling by other molecules. Activation of STING is dependent on the presence of intracellular cGAMP. There are two sources of intracellular cGAMP. First, upon binding to DNA, cGAS is activated to catalyze the synthesis of intracellular cGAMP. Second, extracellular cGAMP is transported into the cell through importers. After cGAMP binds to STING, STING is translocated to the Golgi apparatus to bind sCAGs and is linked to the K63-linked ubiquitination by TRIM32 and TIRM56, which induces STING to recruit TBK1 and activate IRF3-induced IFN-I production. The cGAS/STING cascade is controlled by various factors. For example, DNA-PK, AIM2, miR-23a/b, etc. reduce the activity and expression of cGAS through different mechanisms. However, G3BP1, β-arrestin 2, and hnRNPA2B1 enhance the activity and expression of cGAS through different mechanisms. In addition, the entry of extracellular cGAMP into cells is affected by the trafficking of importers SLC19A1 and SLC46A2, as well as by VRACs and ENPP1. Numerous molecules also regulate the translocation of STING. For example, COPII, YIPF5i, Rhom2, etc., enhance the transport of STING from the ER to the Golgi and perinuclear microsomes. However, MTMR3/4 and STIM1 inhibited the translocation of STING. In addition, the activation of STING are also positively and negatively regulated by various factors, such as KDM5, PPP6C, and EGFR.