Figure 2. Kaplan-Meier Estimates of Overall and Progression-Free Survival.
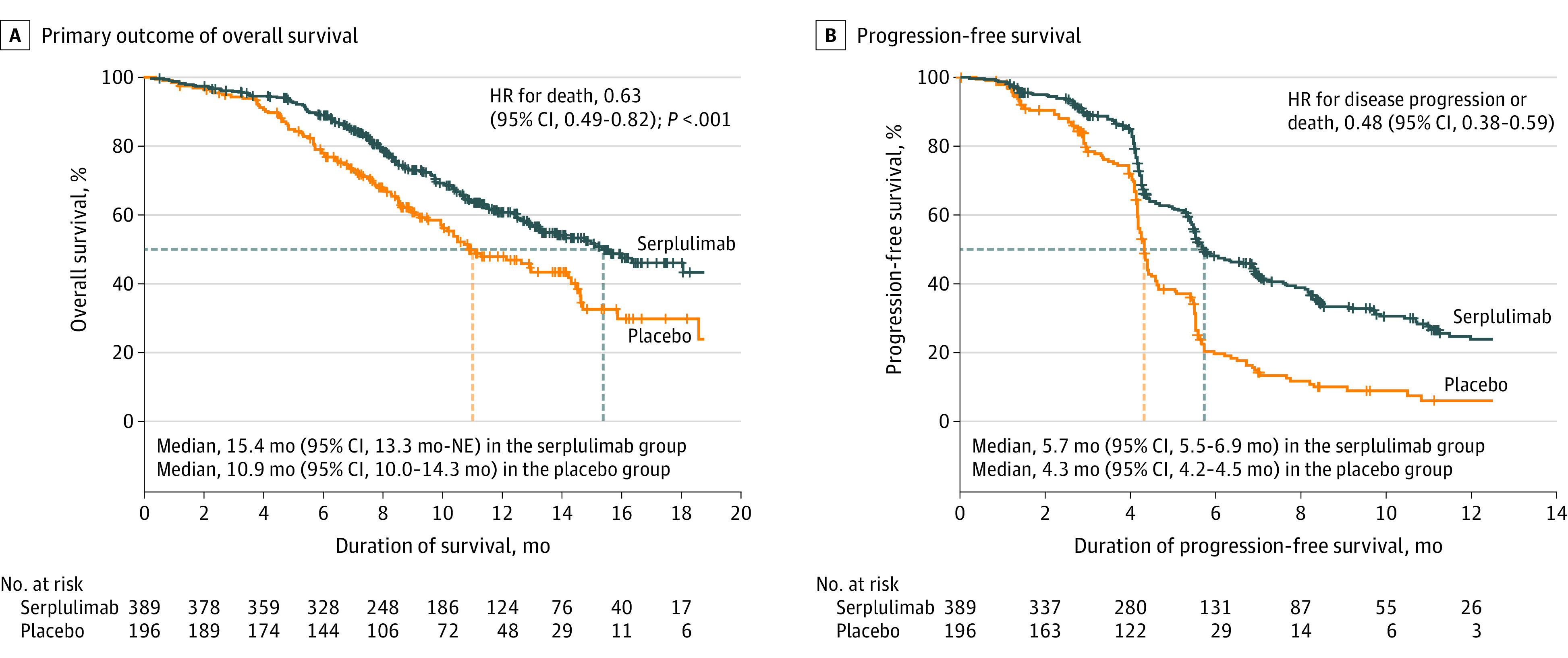
Data are from assessments by the independent radiology review committee using version 1.1 of the Response Evaluation Criteria in Solid Tumors. The tick marks indicate censored data. The median duration of follow-up for overall survival at the interim analysis was 12.5 months (IQR, 8.9-15.5 months) for the serplulimab group and 12.3 months (IQR, 8.6-14.9 months) for the placebo group. The median duration of follow-up for progression-free survival was 9.5 months (IQR, 5.6-13.2 months) for the serplulimab group and 8.4 months (IQR, 7.0-13.0 months) for the placebo group. HR indicates hazard ratio; NE, not evaluable.
