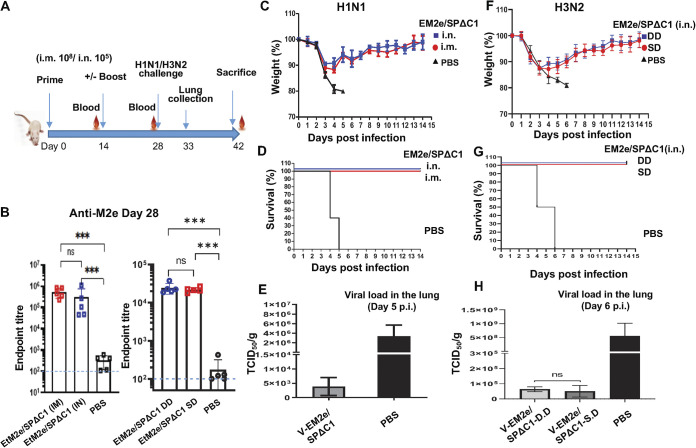
FIG 7.
Mice immunized with V-EM2/SPΔC1 were protected against the lethal challenge of H1N1 and H3N2 influenza viruses. (A) Schematic of the bivalent VSV vaccine candidate immunization and influenza virus challenge protocol used in the study. For the H1N1 challenge experiment, the BALB/c mice were immunized with 1 × 108 TCID50 (IM) or 1 × 105 TCID50 (IN) of V-EM2e/SPΔC1 or PBS on day 0 and day 14. On day 27, the blood samples were collected and measured for anti-influenza M2e antibody levels by ELISA (B). On day 28, all the mice were challenged with 2,100 PFU of H1N1 influenza virus. Weight loss (C) and survival rates (D) of the mice were monitored daily for 2 weeks. (E) Viral loads in the lung tissue of immunized mice and PBS group at day 5 post-H1N1 challenge were measured in MDCK cell line, as described in the Materials and Methods. For the H3N2 challenge experiment, the BALB/c mice were immunized with 1 × 105 TCID50 (IN) of V-EM2e/SPΔC1 or PBS on day 0 (single-dose, SD), or on day 0 and 14 (double-dose, DD). On day 28, all the mice were challenged with 1.4 × 104 PFU of H3N2. (F) Weight loss, (G) survive rates, and (H) viral loads in the lung tissue of immunized mice and PBS group at day 6 after H3N2 challenge.