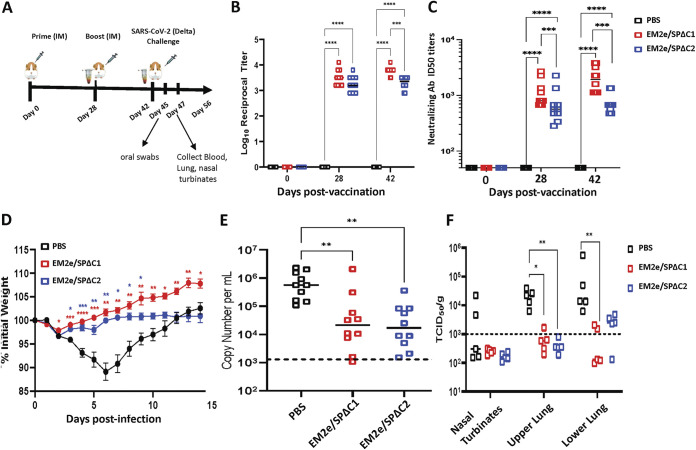
FIG 8.
V-EM2/SPΔC1 and V-EM2/SPΔC2 provided protection against SARS-CoV-2 Delta infection in Syrian Hamsters. (A) Schematic of the bivalent VSV vaccine candidate immunization and SARS-CoV-2 Delta variant challenge protocol used in the study. (B) Total serum anti-SARS-CoV-2 spike IgG titers in hamsters following prime and boost vaccination. (C) The neutralizing antibody titers in immunized mice sera against SARS-CoV-2 Delta variant were measured and neutralizing titers were calculated by using sigmoid 4PL interpolation with GraphPad Prism 9.0, as described in the Materials and Methods. (D) Weight loss in the vaccinated or the control Syrian hamsters following infection with the SARS-CoV-2 Delta variant. (E) Viral RNA levels in oral swabs on day 3 following infection with SARS-CoV-2 Delta variant. (F) Infectious SARS-CoV-2 Delta virus titers in nasal turbinates and lung tissues on day 5 following infection with SARS-CoV-2 delta. n = 10 for B (each time point), n = 10 for C, 10 through day 28, and 10 at day 42; n = 10 for D (at day 3 postinfection) and n = 5 for E (from day 5 postinfection). Statistical significance was assessed by two-way analysis of variance with multiple comparisons (A), mixed effects analysis with multiple comparisons (B), and the Kruskal-Wallis test with multiple comparisons (C and D). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. For (B), colored asterisks indicate significant differences between the same colored group compared with the PBS group. Shown are medians for each group in A, C, and D, and mean + SEM in B.