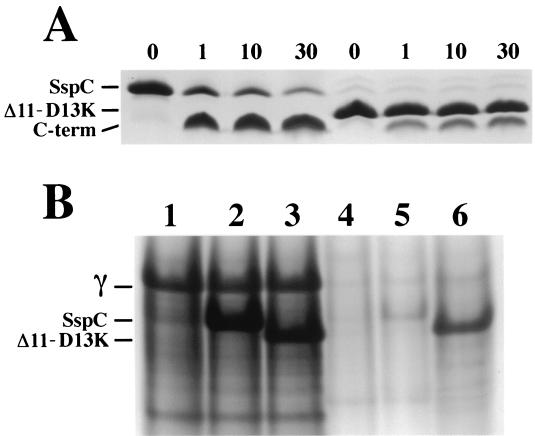
FIG. 4.
Degradation of SspC and SspCΔ11-D13K in vitro and in vivo. (A) SspC and SspCΔ11-D13K (50 μM) were equilibrated with supercoiled pUC19 plasmid DNA (0.13 mg/ml) in 10 mM Tris-HCl (pH 7.4)–150 mM NaCl–2 mM CaCl2 at 37°C for 90 min prior to addition of partially purified B. megaterium GPR to 20 μg/ml. Samples were removed before (0 min) and after addition of GPR (1, 10, and 30 min) for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on Tris-tricine gels (17). The positions of full-length proteins and the C-terminal GPR cleavage peptide (C-term) are indicated on the left of the figure, and the minutes of incubation with GPR are given above the lanes. (B) Total SASP were acid extracted from dry, ruptured dormant spores (lanes 1 through 3) and purified germinated spores (germinated for 100 min as described in the text) (lanes 4 through 6), and samples from equivalent amounts of spores and germinated spores were run on polyacrylamide gel electrophoresis at low pH (15). The samples in each lane are as follows: lane 1, α−β− dormant spores; lane 2, α−β−SspC dormant spores; lane 3, α−β−SspCΔ11-D13K dormant spores; lane 4, α−β− germinated spores; lane 5, α−β−SspC germinated spores; and lane 6, α−β−SspCΔ11-D13K germinated spores. The positions of SASP-γ (γ) SspC, and SspCΔ11-D13K are indicated on the left.