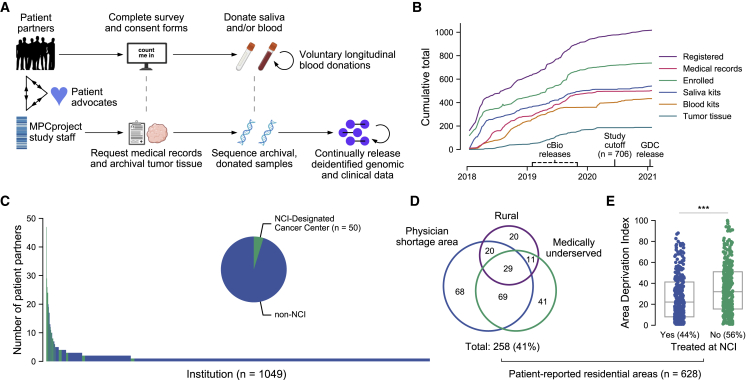
Figure 1.
Partnering with diverse patients to enhance our understanding of metastatic prostate cancer
(A) Summary of MPCproject enrollment process. Patients learn about the project primarily through outreach and partnered advocacy groups. If they register, patient partners complete online intake, consent, and medical release forms, then can opt into donating saliva via a mailed kit and/or blood at routine blood draws at no charge. In parallel, MPCproject staff request medical records and archival tumor samples from patients’ medical institutions, then abstract medical information from obtained records and sequence archival tumor tissue and/or donated blood and saliva (STAR Methods). Deidentified clinical, genomic, and patient-reported data are released on a continual, prepublication basis and deposited in public repositories.
(B) Enrollment statistics and timeline for the MPCproject. Depicted are the cumulative number of patients that began the registration process (registered), patients that completed the survey and consent forms (enrolled), patients with at least one medical record received (medical records), and blood kits, saliva kits, and archival tumor tissue received at the Broad Institute for sequencing (blood kits, saliva kits, and tumor tissue, respectively). 706 patient partners enrolled before “study cutoff,” June 1, 2020, and are included in this study’s analyses. cBioPortal (cbioportal.org) releases include summary abstracted medical, genomic, and patient-reported data; Genomic Data Commons (GDC) releases include raw sequencing files and demographic data.
(C) Represented medical institutions among patient partners living in the US and Canada. Shown are the 1,049 unique institutions (x axis) where patient partners report receiving care for their prostate cancer, with the number of distinct patient partners at each institution (y axis). NCI-designated cancer centers are shown in green. Patient partners that did not complete this survey question (n = 36) and institutions outside the US and Canada (n = 56) are not shown.
(D) Access to medical care among patient partners living in the US. Patient-reported data were used to identify residential census tracts that were overlapped with primary care health-physician-shortage areas (HPSAs), medically underserved population/areas (MUAs), and rural areas obtained from the Health Resources and Services Administration and US Census. Patient partners that live in Canada (n = 30) who did not provide residential data (n = 40) or who provided only a P.O. box (n = 8) are not shown.
(E) Patient partners living in more disadvantaged areas are less likely to attend NCI cancer centers. The Area Deprivation Index, a metric that assesses neighborhood disadvantage, was assessed for each residential census block group. Higher values indicate more disadvantage. The x axis reflects whether patient partners reported receiving care at an NCI-designated cancer center. ∗∗∗ p < 0.001 in a logistic regression model that adjusts for rural, MUA, and HPSA status.