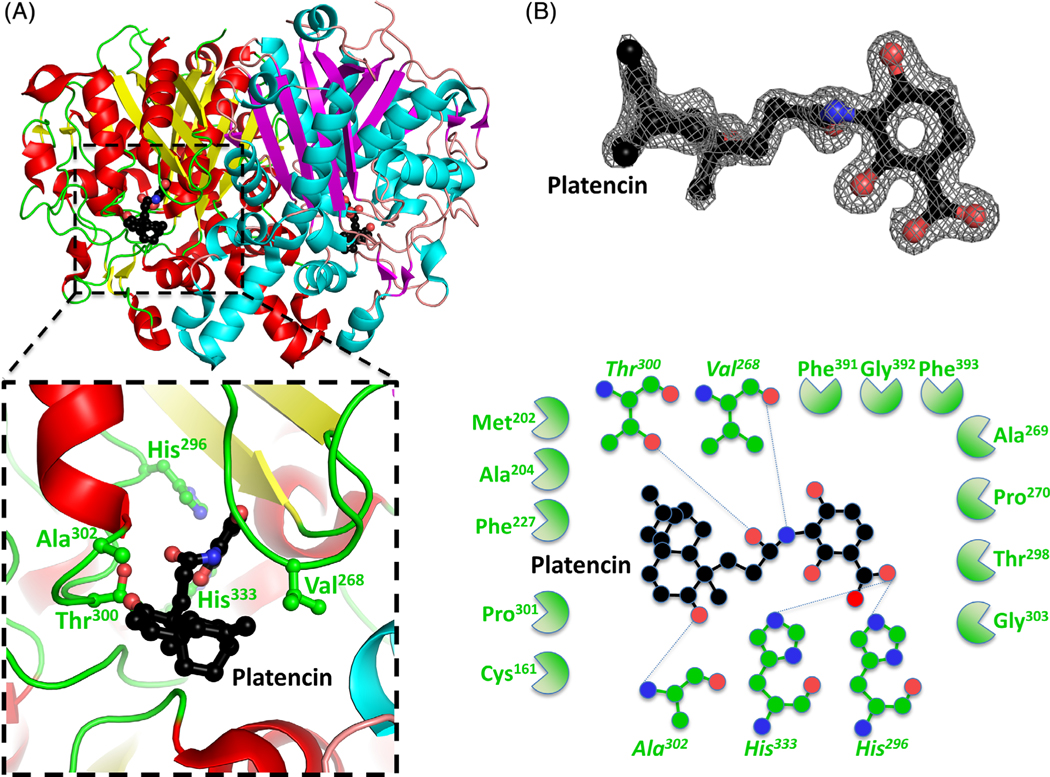
FIGURE 4.
Structure of the β-ketoacyl-ACP synthase I from Brucella melitensis bound to platencin. A, The two monomers are shown in cartoon form, with one monomer colored red (α-helices) and yellow (β-sheets), and the second monomer colored blue (α-helices) and purple (β-sheets). Platencin is colored black (carbon), red (oxygen), and blue (nitrogen). The insert shows the binding pocket and interface residues. B, The location of platencin was supported by good electron density. The electron density map (2Fo-Fc) is contoured to 2 sigma, and colored gray. The full binding pocket and interacting residues are presented in cartoon form. The blue dashed lines indicate hydrogen bonds. The green shaped amino acids indicate hydrophobic interactions [Color figure can be viewed at wileyonlinelibrary.com]