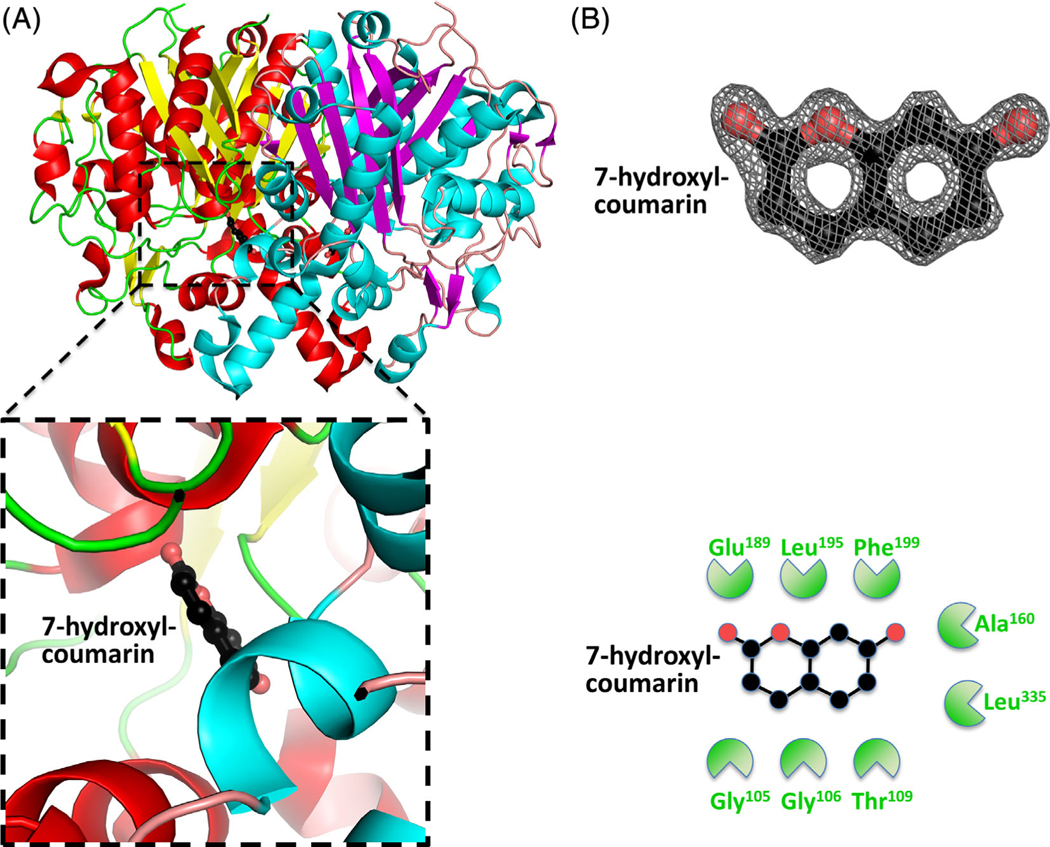
FIGURE 5.
Structure of the β-ketoacyl-ACP synthase I from Brucella melitensis bound to 7-hydroxyl-coumarin. A, The two monomers are shown in cartoon form, with one monomer colored red (α-helices) and yellow (β-sheets), and the second monomer colored blue (α-helices) and purple (β-sheets). 7-Hydroxyl-coumarin is colored black (carbon), red (oxygen), and blue (nitrogen). The insert shows the binding pocket and interface residues. B, The location of 7-hydroxyl-coumarin was supported by good electron density. The electron density map (2Fo-Fc) is contoured to 2 sigma, and colored gray. The full binding pocket and interacting residues are presented in cartoon form. The green shaped amino acids indicate hydrophobic interactions [Color figure can be viewed at wileyonlinelibrary.com]