Abstract
Purpose
Perioperative sleep disorders have attracted much attention due to their high prevalence and severe harm, and the current treatment methods are insufficient. Some randomized controlled trials (RCTs) have produced controversial results on whether melatonin can improve perioperative sleep quality. This review aimed to evaluate the effects of melatonin treatment on perioperative sleep quality.
Patients and Methods
A systematic search of six databases was performed to identify RCTs investigating melatonin and perioperative sleep. The outcomes analyzed were subjective sleep quality, sleep latency (SL), total sleep time (TST), sleep efficiency (SE), the behavior of awakenings and daily naps, and the incidence of poor sleep quality. RevMan 5.4 and Stata 16 software was used for the meta-analysis and sensitivity analysis, and trial sequential analysis was conducted using TSA 0.9.5.10 Beta software. This study was registered in PROSPERO (registration number: CRD42022311378).
Results
10 studies containing 725 participants were included. Melatonin improved postoperative subjective sleep quality (SMD: −0.30; 95% CI: [−0.47, −0.14]; P = 0.0004) but not preoperative sleep quality (MD: −2.76; 95% CI: [−10.44, 4.91]; P = 0.48). In the postoperative period, 6mg dose had the best efficacy (SMD: −0.31; 95% CI: [−0.57, −0.04]; P = 0.02). Melatonin increased postoperative TST (P = 0.02) and SE (P = 0.002) and decreased the incidence of postoperative poor sleep quality (P = 0.002) but had no effect on SL (P = 0.11), the number and duration of awakenings (P = 0.28; P=0.55), and the number and duration of daily naps (P = 0.26; P = 0.38). The trial sequential analysis showed that the accumulated Z value crossed both the traditional boundary value and the TSA boundary value, further confirming the stability of the result of the meta-analysis.
Conclusion
Melatonin treatment can improve postoperative sleep quality. A 6mg daily dose of melatonin may have a better beneficial effect, which needs further exploration. This study supports the application of melatonin for improving postoperative sleep quality.
Keywords: melatonin, sleep quality, perioperative, preoperative, postoperative, meta-analysis
Introduction
Perioperative sleep disorders have become a significant challenge in the current medical environment due to their high prevalence, with estimates ranging from 64% to 73.1%.1–3 Perioperative sleep disorders may contribute to a variety of serious postoperative complications. The common complications include postoperative neurocognitive disorder (PND), postoperative delirium, postoperative pain exacerbation, and sensitization.1,4–7 In addition, mood disorders and other psychological problems, cardiovascular disorders, metabolic impairment, and immune disorders can also occur.4,6 More than that, the length of hospital stay will be extended, and the prognosis of surgical patients will be affected.8,9 However, there is still no definite treatment for perioperative sleep disorders. Treatments that focus on the sleep environment and mood are usually influenced by multiple factors. Traditional drug treatment, such as benzodiazepines, is often limited due to the noticeable adverse reactions.4,6 Although acupuncture has emerged in recent years, it needs to be further confirmed by large-scale trials owing to limited efficacy and lack of evidence.10 Thus, there is an urgent need for a drug with a definite sleep-promoting effect and fewer adverse reactions to improve perioperative sleep quality.
Melatonin (n acetyl 5 methoxytryptamine), an indoleamine produced by the pineal gland, is the neuroendocrine basis for regulating sleep and has a variety of other physiological functions (such as modulating blood pressure, body temperature, immune and metabolic function).11–13 Studies have supported the clinical application of perioperative melatonin as a treatment for sleep disorders, anxiety, and pain.14–17 Many randomized controlled trials (RCTs) demonstrated that melatonin treatment was effective in improving preoperative and postoperative sleep quality.18–20 Besides, melatonin rarely produces dependence and hangover effects compared to traditional sleep medications.21 A systematic review also showed that perioperative melatonin was not associated with any known adverse reactions.22
Considering the high incidence rate and severe consequences of perioperative sleep disorders, many RCTs have explored the association between perioperative sleep quality and melatonin. However, inconsistent or conflicting findings were obtained in some studies.23–25 To clarify these contradictory results and more accurately assess the effectiveness of melatonin, a systematic review and meta-analysis were performed. Interestingly, some studies showed that melatonin did not improve perioperative sleep quality but improved sleep-related indexes such as sleep latency (SL), total sleep time (TST), and sleep efficiency (SE).26,27 In addition, most previous studies had small sample sizes.20,24,26 We therefore systemically searched and analyzed the available literature to assess not only the efficiency of melatonin treatment on perioperative subjective sleep quality but also on the sleep-related indexes, including SL, TST, SE, the number and duration of awakenings, and the number and duration of daily naps. In Andersen’s systematic review,22 the effects of melatonin on perioperative sleep quality were only qualitatively analyzed, not quantitatively. Zhang et al8 also performed a meta-analysis. However, the participants only involved patients undergoing laparoscopic cholecystectomy (LC) and only assessed postoperative sleep quality, and preoperative sleep quality has not been systematically assessed. Only 5 studies were included in Zhang’s study and concluded that melatonin did not improve postoperative sleep quality. Therefore, our study has some unique features. We analyzed the sleep quality of the whole perioperative period (including preoperative and postoperative) and performed a subgroup analysis based on several doses of melatonin. And we also evaluate the efficacy of melatonin treatment on the incidence of postoperative poor sleep quality.
This meta-analysis aimed to evaluate the clinical efficacy of melatonin treatment on perioperative sleep quality and the incidence of poor sleep quality and determine which dose of melatonin shows the greatest benefit.
Materials and Methods
This study has been registered in PROSPERO (registration number: CRD42022311378) and was conducted according to the PRISMA statement.28
Search Strategy
Databases, including PubMed, Embase, Cochrane library, Web of Science, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform, were searched to find relevant articles from the establishment of the database to February 06, 2022, in all languages. The search terms were as follows: 1) sleep, sleep quality, sleep wake disorders, sleep disorder; 2) perioperative, preoperative, postoperative, surgery, operation, anesthesia; 3) melatonin, n acetyl 5 methoxytryptamine; and 4) study type. Then, we checked the lists of references of the relevant articles to find additional articles. Finally, the detailed search strategy of each database was shown in the additional file (see Appendix 1–4).
Eligibility Criteria
According to the PICOS formula, the studies meeting the following criteria were included:
Participants: patients undergoing all types of surgery under any anesthesia.
Interventions: melatonin treatment during the perioperative period with no limitation of dose, route of administration, and time of administration.
Comparisons: Placebo
Outcomes: sleep-related outcome measures were reported from 3 days before surgery to 3 days after surgery. The subjective sleep quality scale, including the visual analog scale (VAS) and sleep quality scale (SQS), were used as the primary outcomes. Sleep latency (SL), total sleep time (TST), sleep efficiency (SE), number and duration of awakenings, number and duration of daily naps, and the number of patients with poor sleep quality were used as the secondary outcomes.
Study Design: RCTs
We excluded the following: 1) articles that were repeatedly published or had no quantitative outcomes; 2) the results were not reported within the range of 3 days before surgery to 3 days after surgery; 3) reviews, case reports, conference abstracts, study protocols, bulletins, and consensus statements.
Study Selection
We used EndNote X9 software to manage the references and remove duplicate articles. The retrieved results were preliminarily screened by reading the title and abstract. If a study was eligible or ambiguous, we read the full text for secondary screening. The process of study selection was performed independently by two investigators (Gao & Chen). Disagreements were resolved through discussion. If the opinions were not unified, the third independent reviewer (Li) participated and decided.
Data Extraction
Two investigators (Gao & Chen) independently extracted related data, including study characteristics and measured outcomes. Disagreements were resolved through consultation with the third investigator (Li). The study characteristics include the first author’s name, year of publication, study location, study design, the type of surgery, sample size, gender, mean age, interventions (dose, route of administration, and time of melatonin), comparisons, reported outcomes type. The measured outcomes include the mean and standard deviation (SD) after the intervention of VAS or SQS score, SL, TST, SE, number and duration of awakenings, and number and duration of daily naps, but also include the number of patients or incidence of perioperative poor sleep quality or perioperative sleep disorders. Then, ClinicalTrials.gov and WHO International Clinical Trials Registry Platform were evaluated to obtain the most recent and complete data. Finally, if the reporting was unclear, we contacted the corresponding author to obtain the complete data.
Risk of Bias
For risk of bias, two independent investigators (Gao & Chen) used the Cochrane Risk of Bias tool (RoB 2.0) to estimate selection, performance, detection, attrition, reporting, and other biases.29 The RoB 2.0 comprises the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. These items were scored as low, unclear, or high risk of bias. Disagreements were resolved through discussion.
Statistical Analysis
The effect of melatonin on perioperative sleep quality was assessed by the mean and SD of the following continuous outcomes: VAS or SQS score, SL, TST, SE, number and duration of awakenings, and number and duration of daily naps. In addition, we used dichotomous data to assess the efficacy of melatonin treatment on the incidence of poor sleep quality. For continuous outcomes, we calculated the mean difference (MD) with 95% confidence intervals (CI). If the data included both VAS and SQS scores, the standardized mean difference (SMD) was used to calculate results with 95% CI. We calculated dichotomous outcomes as the risk ratio (RR) was used with 95% CI. The P-value of less than 0.05 was deemed statistically significant. If the outcomes are reported several times during the preoperative or postoperative period, we calculated the mean value as the overall outcomes. The chi2 test and I2 statistic were used to evaluate heterogeneity across studies. Significant heterogeneity was considered to exist if the chi2 test yielded P < 0.10 and I2 statistic >50%, and the random-effects model (REM) was used. Otherwise, we considered that there is no significant heterogeneity across studies, and the fixed-effects model (FEM) was utilized. Strikingly, to determine the possible sources of heterogeneity across studies and the respective effect of different doses of melatonin on postoperative subjective sleep quality, we performed a subgroup analysis according to several doses of melatonin. Meta-analysis was conducted through the RevMan 5.4 software.
In addition, to further confirm the result of the meta-analysis, the TSA 0.9.5.10 Beta was used for trial sequential analysis. The boundary value type of hypothesis testing is set as a two-sided test. We set the probability of the type I error as α = 0. 05, and the sample size is taken as the expected information value (RIS). Finally, to evaluate the reliability of the result of this meta-analysis, we performed a sensitivity analysis by the Stata 16 software.
Results
Search results
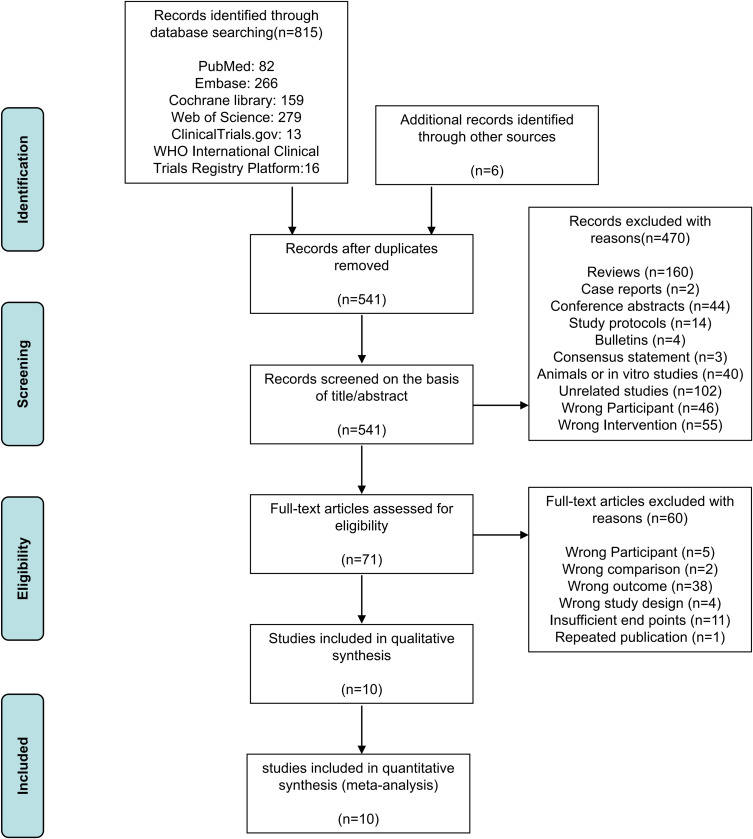
The initial search identified 815 records through PubMed, Embase, Cochrane library, Web of Science, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform, and 6 additional records. 541 records remained after removing 280 duplicates by using EndNote X9. After screening the title and abstract, 470 studies were excluded, and 71 records were included for full-text evaluation. After applying eligibility criteria, 60 studies were excluded due to wrong participant (n = 5), wrong comparison (n = 2), wrong outcome (n = 38), wrong study design (n = 4), Insufficient endpoints (n=11), or repeated publication (n = 1). Finally, 10 trials were selected in this review. The flow diagram of the study screening is shown in Figure 1.
Figure 1.
Flow diagram of study screening.
Characteristics of Studies
Eventually, 10 RCTs were identified, and the total sample size was 725, including 380 participants in the melatonin group and 345 participants in the placebo group. Studies were carried out in various countries such as China,19 Denmark,24–27 India,30 Saudi Arabia,31 Singapore,32 Turkey,20 and the USA.23 The type of surgery included breast Cancer Surgery,26,27 hip arthroplasty,19 knee arthroplasty,23 laparoscopic cholecystectomy (LC),24,25,30 minor elective surgery,31 prostatectomy,20 and wisdom teeth extraction.32 The sample size ranged from 3724 to 139.19 Some studies involved only males20 or females.24,26,27 The rest of the included studies enrolled both genders.19,23,25,30–32 The melatonin dose ranged from 1mg19 to 10mg,24 among which 6 mg was the most frequently used dose.20,26,27,30,32 In addition, Samarkandi’s study31 included more than one melatonin group, ie, one receiving melatonin at a dose of 0.1mg/kg, one receiving melatonin at a dose of 0.25mg/kg, and the other with a dose of 0.5mg/kg. Only Andersen’s study24 used the type of administration of intravenous infusion, and the type of administration of all other studies was oral.19,20,23,25–27,30–32 2 studies26,27 reported the effectiveness of melatonin treatment on preoperative VAS scores. 6 studies19,24–26,30,32 reported the effectiveness of melatonin on postoperative VAS scores. One study23 reported the effectiveness of melatonin on postoperative SQS scores. 3 studies25,26,30 reported the efficacy of melatonin on SL, TST, number of awakenings, and number of daily naps. 2 studies26,27 reported the efficacy of melatonin on SE. 2 studies25,30 reported the efficacy of melatonin on the duration of awakenings and duration of daily naps. 2 studies20,31 reported the efficacy of melatonin on the incidence of poor postoperative sleep quality. The detailed characteristics of these 10 studies are shown in Table 1.
Table 1.
Characteristics of the Studies
| Study (Country) | Participants (the Type of Surgery) | Sample Size, Gender, Age (Melatonin/Placebo) | Interventions (Daily Dose, Route of Administration, Time) | Outcomes |
|---|---|---|---|---|
| Andersen 201424 (Denmark) | LC | MEL: 20, all female, 57 (23–69) PLA: 21, all female, 45 (20–67) Data are median and range |
MEL-10mg (25 mL ethanol/saline solution containing 10 mg of melatonin), intravenous infusion, the time of surgical incision |
VAS |
| Borazan 201020 (Turkey) | Prostatectomy | MEL: 26, all male, 57 ± 7 PLA: 26, all male, 58 ± 6 Data are mean ± SD |
MEL tablet-6mg, oral, PERD1 (11 p.m.) and 1 h before surgery |
Incidence of poor sleep quality |
| Fan 201719 (China) | Hip arthroplasty | MEL: 69, 25 male/44 female, 74.6 ± 5.4 PLA: 70, 28 male/42 female, 74.5 ± 5.7 Data are mean ± SD |
MEL preparation-1mg, oral, PERD1 (1 h before sleep) and POD1-5 |
VAS |
| Gögenur 200925 (Denmark) | LC | MEL: 60, 19 male/41 female, 44 ± 13 PLA: 61, 16 male/45 female, 47 ± 12 Data are mean ± SD |
MEL capsule-5mg, oral, 30 min before sleep in POD1-3 |
VAS SL, TST, number and duration of awakenings, number and duration of daily naps |
| Hansen 201427 (Denmark) | Breast cancer surgery | MEL: 28, all female, 51 (46–66) PLA: 26, all female, 60 (49–68) Data are median and 25–75% IQR |
MEL tablet-6mg, oral, 1 h before sleep from PERD7 to 12 weeks after surgery |
VAS SE |
| Kirksey 201523 (USA) | Knee arthroplasty | MEL: 19, 5 male/14 female, 70 ± 9.3 PLA: 18, 12 male/6 female, 61.4 ± 14.3 Data are mean ± SD |
MEL tablet-5mg, oral, shortly before sleep for 7 days from PERD3 to POD3 |
SQS |
| Madsen 201626 (Denmark) | Breast cancer surgery | MEL: 27, all female, 51 (38–74) PLA: 21, all female, 59 (34–74) Data are median and range |
MEL tablet-6mg, oral, 1 h before sleep from PERD3 to 12 weeks after surgery |
VAS SL, TST, SE, number of awakenings, number of daily naps |
| Samarkandi 200531 (Saudi Arabia) | Minor elective surgery (inguinal hernia, undescended testis, hydrocoele and hypospadias) |
MEL1: 15, 8 male/7 female, 3.9 (3.1–4.6) MEL2: 15, 9 male/6 female, 3.7 (3–4.3) MEL3: 15, 12 male/3 female, 3.1 (2.7–3.6) PLA: 15, 9 male/6 female, 3.6 (2.9–4.2) Data are median and 25–75% IQR |
MEL1: 0.1mg/kg MEL2: 0.25mg/kg MEL3: 0.5mg/kg, oral, 1 h before surgery |
Incidence of sleep disorders |
| Seet 201532 (Singapore) | Wisdom teeth extraction | MEL: 36, 24 male/12 female, 22.7 ± 2.2 PLA: 37, 23 male/14 female, 23.0 ± 2.8 Data are mean ± SD |
MEL tablet-6mg, oral, 90 min before surgery |
VAS |
| Vij 201830 (India) | LC | MEL: 50, 13 male/37 female, 42.8 ± 9.81 PLA: 50, 9 male/41 female, 41.4 ± 10.85 Data are median ± SD |
MEL tablet-3mg*2, oral, 45 min before sleep for POD1-3 |
VAS SL, TST, number and duration of awakenings, number and duration of daily naps |
Note: Comparisons were all placebo and not listed in Table 1.
Abbreviations: LC, laparoscopic cholecystectomy; MEL, melatonin; PLA, placebo; SD, standard deviation; IQR, interquartile range; PERD1, 1 day before surgery; PERD3, 3 days before surgery, PERD7, 7 days before surgery; POD3 3 days after surgery; POD1-3, 1 to 3 days after surgery; POD1-5, 1 to 5 days after surgery; SL, sleep latency; TST, total sleep time; SE, sleep efficiency.
Risk of Bias
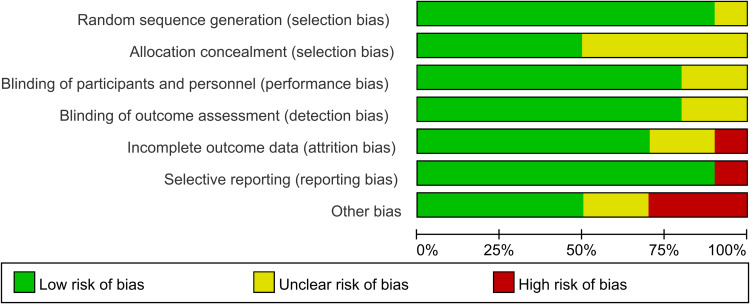
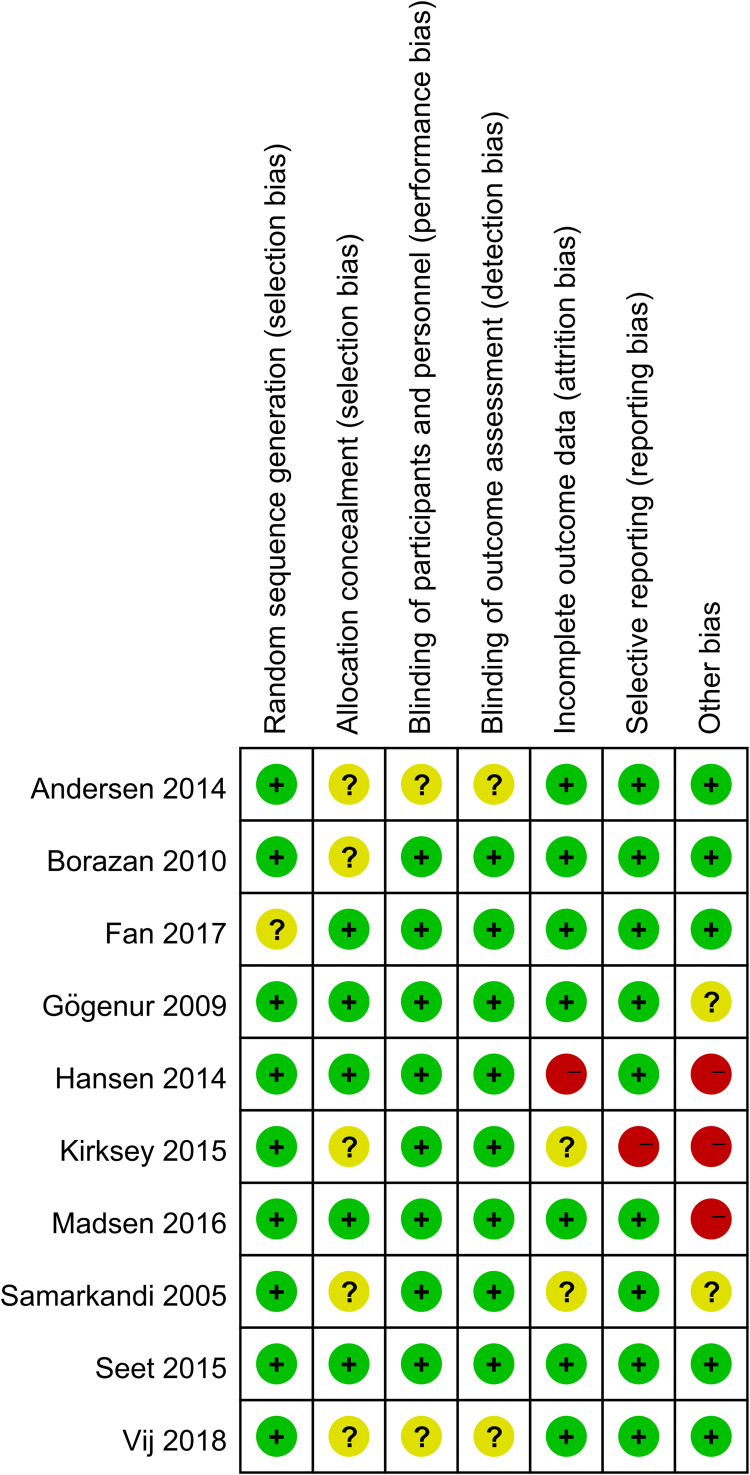
10 studies were assessed for the risk of bias, the abridged risk of bias graph is shown in Figure 2, and the risk of bias details of each study are described in Figure 3. One study19 provided insufficient details about generating random sequences and was considered an unclear risk of bias, and the other 9 studies20,23–27,30–32 showed low risk of bias. 5 studies20,23,24,30,31 assessed the unclear risks of selection bias for the insufficient information on the allocation concealment. 8 studies19,20,23,25–27,31,32 showed low risk of performance bias and detection bias in terms of blinding of participants and personnel, and the remaining 2 studies24,30 apprised as unclear risks of performance bias and detection bias due to lack of the relevant details. Due to the incomplete outcome data, one study27 was considered at high risk of attrition bias, and 2 studies23,31 showed unclear risks of attrition bias. One study23 showed a high risk of reporting bias owing to the selective reporting of pre-stated outcomes. One study27 was terminated prematurely due to an overall low inclusion rate. Therefore, we considered this study to have a high risk of other biases. One study23 showed a higher risk of other biases because of the report of potential conflicts and uneven gender distribution of baseline. We considered the risk of other biases to be high in one study26 due to differences in the median duration of surgery and anesthesia. 2 studies25,31 reported an unclear risk of other bias for the insufficient details about potential conflicts of interest or study fundings.
Figure 2.
Risk of bias graph.
Figure 3.
Risk of bias summary.
Meta-Analysis
Effects of Melatonin Treatment on Postoperative Subjective Sleep Quality
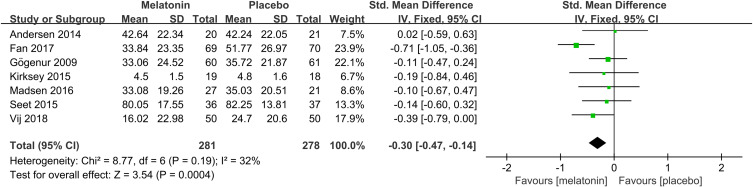
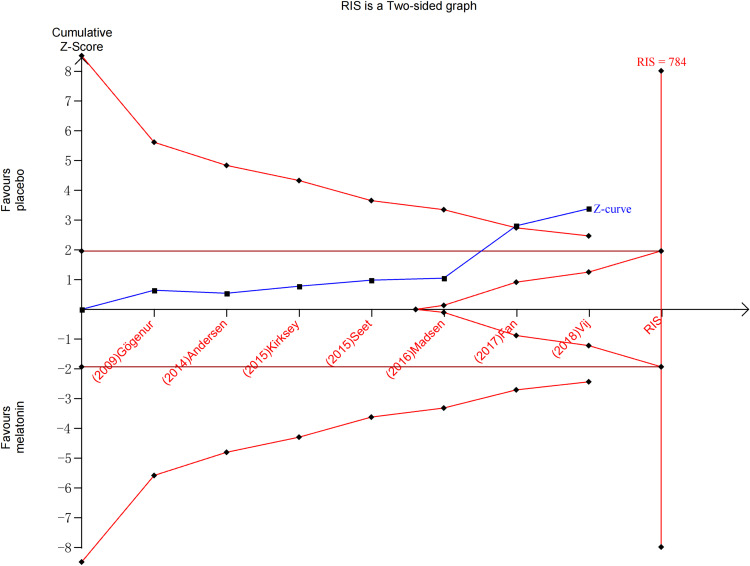
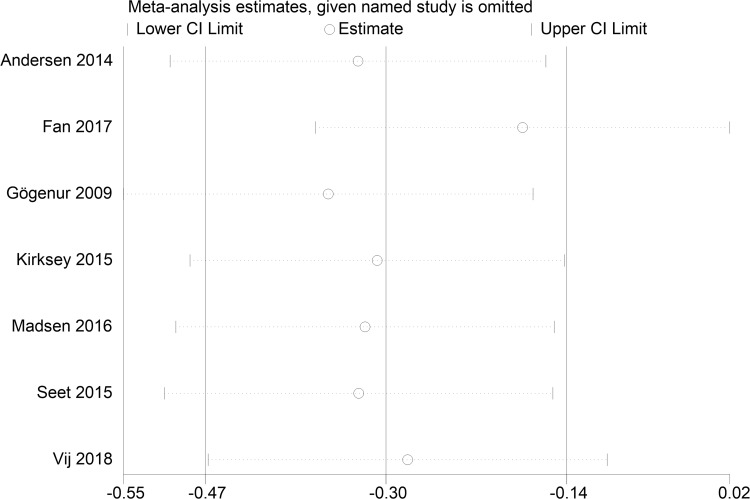
Subjective sleep quality was the primary outcome of the current review. 7 studies19,23–26,30,32 evaluated the effect of melatonin on postoperative subjective sleep quality. Pooling the results of these 7 studies showed that melatonin treatment significantly improved postoperative subjective sleep quality (SMD: −0.30; 95% CI: [−0.47, −0.14]; P = 0.0004) (see Figure 4). The heterogeneity between the studies was low (P = 0.19, I2 = 32%) (see Figure 4). The result of trial sequential analysis showed that the cumulative Z-curve crossed both the conventional boundary and the trial sequential monitoring boundary, although the actual sample size did not exceed the required sample size, which suggested that the result of this meta-analysis is robust, and further confirmed the efficacy of melatonin (see Figure 5). The sensitivity analysis suggested that removing any of these 7 studies did not substantially change the result of the meta-analysis (see Figure 6).
Figure 4.
Forest plot assessing the effect of melatonin treatment on postoperative subjective sleep quality.
Abbreviations: CI, confidence interval; SD, standard deviation; IV, Inverse Variance.
Figure 5.
Trial sequential analysis of the efficacy of melatonin on improving postoperative subjective sleep quality.
Figure 6.
Sensitivity analysis of the effect of melatonin on postoperative subjective sleep quality.
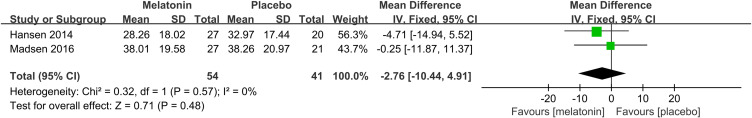
Effects of Melatonin Treatment on Preoperative Subjective Sleep Quality
2 studies26,27 assessed the effectiveness of melatonin treatment on preoperative subjective sleep quality. Pooled analysis of these two studies suggested that melatonin treatment did not improve preoperative subjective sleep quality (MD: −2.76; 95% CI: [−10.44, 4.91]; P = 0.48) (Figure 7). No significant heterogeneity was present among studies (P=0.57, I2 = 0%) (see Figure 7).
Figure 7.
Forest plot assessing the effect of melatonin treatment on preoperative subjective sleep quality.
Abbreviations: CI, confidence interval; SD, standard deviation; IV, Inverse Variance.
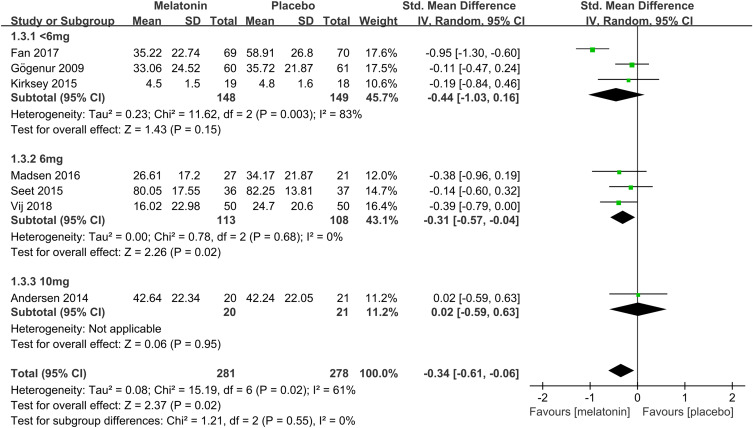
Subgroup Analysis of the Effects of Different Doses of Melatonin on Postoperative Subjective Sleep Quality
To determine the efficacy of different doses of melatonin on postoperative subjective sleep quality, we performed a subgroup analysis based on several doses of melatonin. 3 studies19,23,25 assessed the efficacy of <6mg dose of melatonin on postoperative subjective sleep quality. Pooled analysis of these 3 studies suggested that <6mg dose of melatonin did not improve postoperative subjective sleep quality (SMD: −0.44; 95% CI: [−1.03, 0.16]; P = 0.15) (see Figure 8). There was significant heterogeneity among the studies (P = 0.003, I2 = 83%) (see Figure 8). 3 studies26,30,32 assessed the efficacy of a 6mg dose of melatonin on postoperative subjective sleep quality. Pooled analysis of these 3 studies showed that a 6mg dose of melatonin improved postoperative subjective sleep quality (SMD: −0.31; 95% CI: [−0.57, −0.04]; P = 0.02) (see Figure 8). There was no significant heterogeneity among the studies (P = 0.68, I2 = 0%) (see Figure 8). One study24 assessed the effectiveness of a 10mg dose of melatonin on postoperative subjective sleep quality. The result showed that 10mg dose of melatonin had no meaningful effect (SMD: 0.02; 95% CI: [−0.59, 0.63]; P = 0.95) (see Figure 8).
Figure 8.
Forest plot of subgroup analysis determining the effectiveness of different doses of melatonin on postoperative subjective sleep quality.
Abbreviations: CI, confidence interval; SD, standard deviation; IV, Inverse Variance.
Effects of Melatonin Treatment on Postoperative SL, TST, and SE
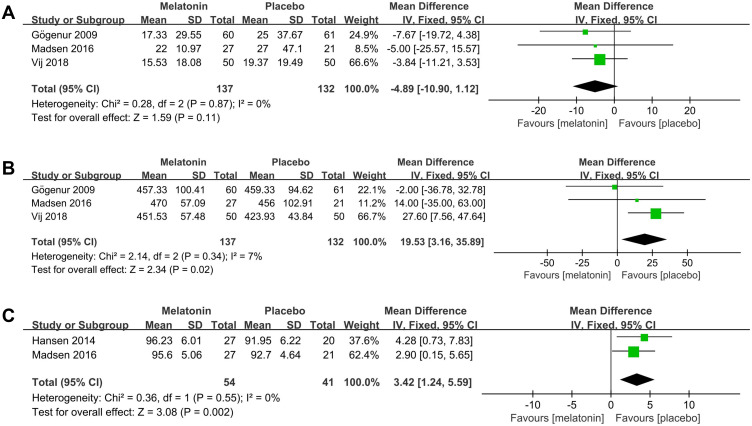
3 studies25,26,30 evaluated the efficacy of melatonin treatment on postoperative SL. Pooled analysis of these 3 studies suggested that melatonin treatment did not decrease postoperative SL (MD: −4.89; 95% CI: [−10.90, 1.12]; P = 0.11) (see Figure 9A). There was no significant heterogeneity among the studies (P = 0.87, I2 = 0%) (see Figure 9A).
Figure 9.
Forest plot assessing the effect of melatonin treatment on postoperative SL, TST and SE. (A) SL. (B) TST. (C) SE.
Abbreviations: CI, confidence interval; SD, standard deviation; IV, Inverse Variance.
3 studies25,26,30 evaluated the efficacy of melatonin treatment on postoperative TST. Pooled analysis of these 3 studies showed that melatonin treatment significantly increased postoperative TST (MD: 19.53; 95% CI: [3.16, 35.89]; P = 0.02) (see Figure 9B). The heterogeneity among the studies was low (P = 0.34, I2 = 7%) (see Figure 9B).
Two studies26,27 evaluated the efficacy of melatonin treatment on postoperative SE. The results obtained by pooling these two studies showed that melatonin treatment significantly increased postoperative SE (MD: 3.42; 95% CI: [1.24, 5.59]; P = 0.002) (see Figure 9C). No significant heterogeneity was present among studies (P = 0.55, I2 = 0%) (see Figure 9C).
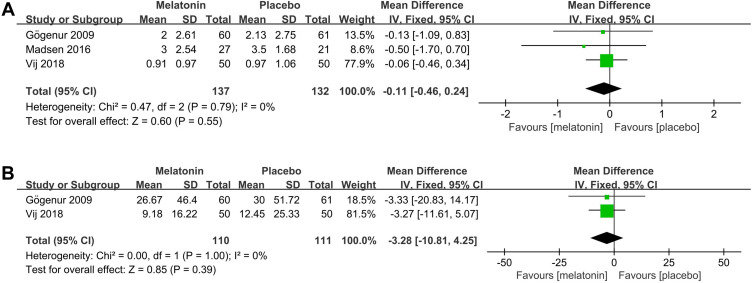
Effects of Melatonin Treatment on the Number and Duration of Awakenings During the Postoperative Period
3 studies25,26,30 assessed the efficacy of melatonin on the number of awakenings during the postoperative period. Pooled analysis of these 3 studies showed that melatonin treatment did not decrease the number of awakenings during the postoperative period (MD: −0.11; 95% CI: [−0.46, 0.24]; P = 0.55) (Figure 10A). No significant heterogeneity was present among studies (P = 0.60, I2 = 0%) (see Figure 10A).
Figure 10.
Forest plot assessing the effect of melatonin treatment on number and duration of awakenings during postoperative period. (A) number of awakenings. (B) duration of awakenings.
Abbreviations: CI, confidence interval; SD, standard deviation; IV, Inverse Variance.
Two studies25,30 assessed the efficacy of melatonin on the duration of awakenings during the postoperative period. Pooled analysis of these two studies showed that melatonin treatment did not decrease the duration of awakenings (MD: −3.28; 95% CI: [−10.81, 4.25]; P = 0.39) (see Figure 10B). No significant heterogeneity was present between the studies (P = 1.00, I2 = 0%) (see Figure 10B).
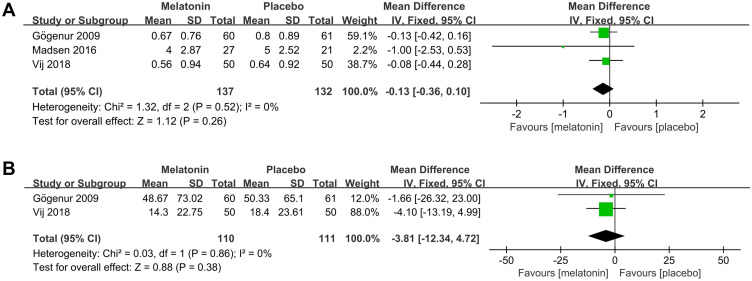
Effects of Melatonin Treatment on the Number and Duration of Daily Naps During the Postoperative Period
3 studies25,26,30 assessed the effect of melatonin on the number of daily naps during the postoperative period. The meta-analysis showed that melatonin had no effect on the number of daily naps during the postoperative period (MD: −0.13; 95% CI: [−0.36, 0.10]; P = 0.26) (see Figure 11A). There was no significant heterogeneity among the studies (P = 0.52, I2 = 0%) (see Figure 11A).
Figure 11.
Forest plot assessing the effect of melatonin treatment on number and duration of daily naps during postoperative period. (A) number of daily naps. (B) duration of daily naps.
Abbreviations: CI, confidence interval; SD, standard deviation; IV, Inverse Variance.
2 studies25,30 assessed the effect of melatonin on the duration of daily naps during the postoperative period. The meta-analysis showed that melatonin had no effect on the duration of daily naps during the postoperative period (MD: −3.81; 95% CI: [−12.34, 4.72]; P = 0.38) (see Figure 11B). There was no significant heterogeneity among the studies (P = 0.86, I2 = 0%) (see Figure 11B).
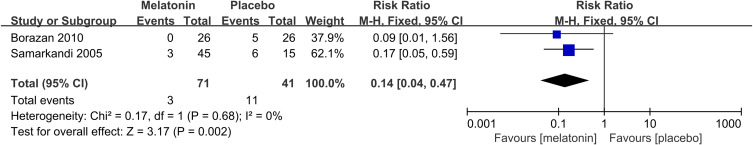
Effects of Melatonin Treatment on the Incidence of Postoperative Poor Sleep Quality
2 studies20,31 reported the incidence of postoperative poor sleep quality. The meta-analysis suggested that melatonin treatment significantly decreased the incidence of postoperative poor sleep quality (RR: 0.14; 95% CI: [0.04, 0.47]; P = 0.002) (see Figure 12). There was no significant heterogeneity among the studies (P = 0.68, I2 = 0%) (see Figure 12).
Figure 12.
Forest plot assessing the effectiveness of melatonin treatment on the incidence of postoperative poor sleep quality.
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
Discussion
Main Findings
In this study, we conducted a meta-analysis of 725 subjects in 10 studies. We found that melatonin treatment significantly improved postoperative subjective sleep quality and decreased the incidence of postoperative poor sleep quality but did not improve preoperative subjective sleep quality. In terms of improving postoperative sleep quality, 6mg of melatonin has the best efficacy, but this needs further elaboration. In addition, melatonin treatment increased postoperative TST and SE but had no effect on postoperative SL, the number and duration of awakenings, and the number and duration of daily naps. Our findings provide evidence support for melatonin in improving postoperative sleep quality.
Interpretation of Results
Subjective Sleep Quality
Our study indicates that melatonin treatment can improve postoperative subjective sleep quality but has no effect on preoperative subjective sleep quality, which is consistent with recent works.18,19,30 Studies indicated that perioperative sleep disorders are the result of the decrease of melatonin concentration in the body, which suggests why melatonin supplementation can improve sleep quality.33,34 Meanwhile, our findings also verify the role of melatonin in regulating the sleep-wake cycle and enhancing darkness-related behavior.35 Notably, two studies24,32 did not use melatonin during the preoperative period but only started on a surgical day and concluded that melatonin did not improve postoperative sleep quality. However, in Fan’s study19 and Borazan’s study,20 melatonin has been used since before surgery, and finally came to the conclusion that melatonin treatment worked, which shows that the duration of melatonin treatment is also critical and suggests that the use of melatonin from preoperative to postoperative may obtain a better sleep promoting effect.
Our study also found that a 6mg daily dose of melatonin had the best efficacy on postoperative sleep quality. However, the 6 mg dose of melatonin was more frequently used in the postoperative period and, therefore, easier to prove its effectiveness. It does not mean that lower or higher doses are not or less effective. Therefore, the effects of different doses of melatonin on postoperative sleep quality still need further exploration.
Sleep-Related Indexes and the Incidence of Poor Sleep Quality
The explanations for the results of sleep-related indexes and the incidence of poor sleep quality in our study are as follows.
Firstly, our study indicates that melatonin can improve postoperative TST and SE but has no effect on SL and the behavior of awakenings and daily naps, which is consistent with previous studies.25–27,30 Although the participants took melatonin, risk factors such as postoperative pain, bright lights, and the noisy environment still exist, which makes it difficult for the patient to fall asleep,6,36 and the efficacy of melatonin on SL will also be affected to a certain extent. As for the behavior of awakenings and daily naps, although melatonin is ineffective, this may not have a significant impact on the overall sleep quality.
Secondly, this study also suggests that melatonin treatment can decrease the incidence of postoperative poor sleep quality. However, de Carvalho’s study18 reported the incidence of preoperative poor sleep quality and concluded that melatonin treatment was ineffective, which matches the results in Figures 4 and 7. In our study, we extracted the number of sleep disorders in Samarkandi’s study31 as the number of poor sleep quality. In fact, some patients without sleep disorders may already have poor sleep quality.37 Therefore, the way we extract data may lead to an inaccurate result, and the results need more research to support due to including only 2 studies and 112 participants.
Strengths of the Study
The strength of this study is that it is the first meta-analysis to assess the effect of melatonin on sleep quality during the whole perioperative period in all surgical patients. The previous systematic review and meta-analysis had some limitations. Andersen’s work22 systematically reviewed the effectiveness of perioperative melatonin on sleep quality but no quantitative meta-analysis. In Zhang’s meta-analysis,8 the type of surgery only included LC, and only the postoperative sleep quality was analyzed, but not the preoperative. The current review not only involved all types of surgery but also analyzed the sleep quality during the whole perioperative period (including preoperative and postoperative) and included more citations than the previous meta-analysis. In addition, there is currently no meta-analysis that assessed the efficacy of melatonin on perioperative objective sleep-related indexes. Therefore, this meta-analysis also summarizes and analyzes the studies that reported the outcomes of SL, TST, SE, and the behavior of awakenings and daily naps, which can more comprehensively and objectively reflect the effectiveness of melatonin. Notably, considering the possibility that some studies only reported the incidence of poor sleep quality but did not report specific quantitative sleep outcomes. We also pooled the results of these studies and assessed the incidence of postoperative poor sleep quality to include more extensive studies and make a more comprehensive evaluation of the efficacy of melatonin treatment. In order to determine which dose of melatonin has the best benefit on postoperative sleep quality, we also conducted a subgroup analysis based on several doses of melatonin, which provides a guiding value for the clinical application of melatonin to improve postoperative sleep quality. More importantly, to further confirm the result of the meta-analysis, we also conducted a trial sequential analysis. Thus, this review is more valuable than the previous meta-analysis.
Limitations
There are also some limitations in this study. (1) Although this study concluded that melatonin could not improve preoperative sleep quality, this result may be unreliable. Preoperative treatment was studied less than postoperative, which may lead to the generation of bias. Therefore, this question remains that the effects of melatonin on preoperative sleep quality are still uncertain; (2) Only 3 or 2 studies were pooled in the analysis of sleep-related indexes and the incidence of poor sleep quality, which requires more RCT to explore these outcome measures; (3) Although 10 studies were included, we were unable to use the funnel plot to test the possibility of publication bias, as 7 studies were summarized that focus on postoperative subjective sleep quality. For this reason, we did not conduct quantitative statistical analysis of the plots using Egger’s test; (4) The heterogeneity among studies in Figure 8 and the source cannot be determined, despite the usage of the subgroup analysis and REM; (5) The trial sequential analysis showed that the actual sample size did not reach the required sample size, suggesting that more studies need to be included; (6) We did not extract the duration of melatonin treatment for statistical analysis, because the duration of melatonin varies from study to study. As a result, the effectiveness of different duration of melatonin treatment cannot be evaluated. In fact, the duration of melatonin treatment is as important as the dose of melatonin in improving sleep quality; (7) In our study, subjective scales such as vas and SQS were used to assess sleep quality due to the lack of the study using other more accurate methods. However, changes in subjectively perceived sleep quality do not necessarily match the changes in subjective sleep quantity as the former refers inter-alia to a personal feeling of how restorative the sleep was, which may lead to some impact on the results. In the measurement of sleep, PSG has always been considered the gold-standard technique. However, PSG is not commonly used in the clinic due to its cost, technical training, time-consuming and other problems, and it may be disruptive to the sleep of patients due to its cumbersome procedures.38 In addition, although PSQI is considered to be a reliable method, there is a lack of studies using PSQI to assess the effects of melatonin on perioperative sleep quality.39 However, considering the advantages and clinical practicability of vas and SQS, this study can still provide evidence from the perspective of evidence-based medicine for the improvement of sleep quality. (8) This meta-analysis only discusses whether melatonin can improve sleep quality based on the longitudinal dimension without exploring more problems in the horizontal dimensions, so it is unclear whether the effects of melatonin were clinically meaningful. For example, indications, the optimization of the treatment plan, and other issues should also be proposed according to the positioning of exploratory research. However, meta-analysis seems to be powerless for these problems. On the contrary, systematic review or conventional traditional review has more advantages.
Future Research Directions
(1) Future research should pay more attention to preoperative sleep quality to compensate for the lack of studies on preoperative treatment. And future research should also focus on sleep-related indexes. (2) In particular, it is hoped that future studies can explore the respective effects of different doses of melatonin, given the paucity of studies with varying melatonin doses, to provide a guiding clue to the clinical use of melatonin. (3) It is hoped that future studies will adjust for the duration of melatonin treatment to determine which duration of melatonin treatment has the best sleep promoting effect on perioperative sleep quality so as to guide the clinical application of melatonin better. (4) Future research should not only focus on whether melatonin can improve perioperative sleep quality but also explore the impact on the prognosis or whether it can bring clinical benefits after improving sleep quality by using melatonin to determine the far-reaching clinical significance of melatonin. (5) Finally, it is particularly hoped that large-sample multicenter RCTs can be carried out in the future.
Conclusion
In conclusion, melatonin treatment can improve postoperative sleep quality, but not preoperative sleep quality. Moreover, a 6mg daily dose of melatonin may have a better efficacy on postoperative sleep quality, but this still needs further confirmation. Melatonin treatment can also improve postoperative TST and SE and reduce the incidence of postoperative poor sleep quality. Therefore, our study supports using melatonin as an effective treatment for improving postoperative sleep quality.
Acknowledgments
The authors would like to thank the support of Inner Mongolia Autonomous Region Health Science and Technology Plan Project (202202327) and Chifeng Natural Science Foundation (SZR2022217). Additionally, Yi Gao thanked Shuwen Wang very much for her help in graphic production.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, writing, revising, and critically reviewing the article; reviewed and gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agreed to take responsibility and be accountable for all aspects of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang X, Hua D, Tang X, et al. The role of perioperative sleep disturbance in postoperative neurocognitive disorders. Nat Sci Sleep. 2021;13:1395–1410. doi: 10.2147/NSS.S320745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seid Tegegne S, Fenta Alemnew E. Postoperative poor sleep quality and its associated factors among adult patients: a multicenter cross-sectional study. Ann Med Surg. 2022;74:103273. doi: 10.1016/j.amsu.2022.103273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjurström MF, Irwin MR, Bodelsson M, Smith MT, Mattsson-Carlgren N. Preoperative sleep quality and adverse pain outcomes after total hip arthroplasty. Eur J Pain. 2021;25(7):1482–1492. doi: 10.1002/ejp.1761 [DOI] [PubMed] [Google Scholar]
- 4.Hillman DR. Postoperative sleep disturbances: understanding and emerging therapies. Adv Anesth. 2017;35(1):1–24. doi: 10.1016/j.aan.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. 2018;46(12):e1204–e1212. doi: 10.1097/CCM.0000000000003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18(3):273–282. doi: 10.1016/j.smrv.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Orbach-Zinger S, Fireman S, Ben-Haroush A, et al. Preoperative sleep quality predicts postoperative pain after planned caesarean delivery. Eur J Pain. 2017;21(5):787–794. doi: 10.1002/ejp.980 [DOI] [PubMed] [Google Scholar]
- 8.Wang JP, Lu SF, Guo LN, Ren CG, Zhang ZW. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery: a prospective cohort study. Medicine. 2019;98(44):e17708. doi: 10.1097/MD.0000000000017708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo ZY, Li LL, Wang D, Wang HY, Pei FX, Zhou ZK. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res. 2019;14(1):378. doi: 10.1186/s13018-019-1446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Liu Y, Yuan Y, et al. Effects of general versus subarachnoid anaesthesia on circadian melatonin rhythm and postoperative delirium in elderly patients undergoing hip fracture surgery: a prospective cohort clinical trial. EBioMedicine. 2021;70:103490. doi: 10.1016/j.ebiom.2021.103490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posadzki PP, Bajpai R, Kyaw BM, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018;16(1):18. doi: 10.1186/s12916-017-1000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi AV, Mosser EA, Oikonomou G, Prober DA. Melatonin is required for the circadian regulation of sleep. Neuron. 2015;85(6):1193–1199. doi: 10.1016/j.neuron.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen LP. The analgesic effects of exogenous melatonin in humans. Acta Anaesthesiol Scand. 2016;60(7):1024–1025. doi: 10.1111/aas.12747 [DOI] [PubMed] [Google Scholar]
- 14.Yousaf F, Seet E, Venkatraghavan L, Abrishami A, Chung F, Warner DS. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period: a qualitative systematic review of randomized trials. Anesthesiology. 2010;113(4):968–976. doi: 10.1097/ALN.0b013e3181e7d626 [DOI] [PubMed] [Google Scholar]
- 15.Caumo W, Levandovski R, Hidalgo MP. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double-blind, randomized, placebo-controlled study. J Pain. 2009;10(1):100–108. doi: 10.1016/j.jpain.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 16.Caumo W, Torres F, Moreira NL Jr, et al. The clinical impact of preoperative melatonin on postoperative outcomes in patients undergoing abdominal hysterectomy. Anesth Analg. 2007;105(5):1263–1271. doi: 10.1213/01.ane.0000282834.78456.90 [DOI] [PubMed] [Google Scholar]
- 17.Madsen BK, Zetner D, Møller AM, Rosenberg J. Melatonin for preoperative and postoperative anxiety in adults. Cochrane Database Syst Rev. 2020;12(12):Cd009861. doi: 10.1002/14651858.CD009861.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Carvalho Nogueira EF, de Oliveira Vasconcelos R, Teixeira Correia SS, Souza Catunda I, Amorim JA, Do Egito Cavalcanti Vasconcelos B. Is there a benefit to the use of melatonin in preoperative zygomatic fractures? J Oral Maxillofac Surg. 2019;77(10):2017–e1. doi: 10.1016/j.joms.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 19.Fan Y, Yuan L, Ji M, Yang J, Gao D. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: a randomized controlled trial. J Clin Anesth. 2017;39:77–81. doi: 10.1016/j.jclinane.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 20.Borazan H, Tuncer S, Yalcin N, Erol A, Otelcioglu S. Effects of preoperative oral melatonin medication on postoperative analgesia, sleep quality, and sedation in patients undergoing elective prostatectomy: a randomized clinical trial. J Anesth. 2010;24(2):155–160. doi: 10.1007/s00540-010-0891-8 [DOI] [PubMed] [Google Scholar]
- 21.Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen LP, Werner MU, Rosenberg J, Gögenur I. A systematic review of peri-operative melatonin. Anaesthesia. 2014;69(10):1163–1171. doi: 10.1111/anae.12717 [DOI] [PubMed] [Google Scholar]
- 23.Kirksey MA, Yoo D, Danninger T, Stundner O, Ma Y, Memtsoudis SG. Impact of melatonin on sleep and pain after total knee arthroplasty under regional anesthesia with sedation: a double-blind, randomized, placebo-controlled pilot study. J Arthroplasty. 2015;30(12):2370–2375. doi: 10.1016/j.arth.2015.06.034 [DOI] [PubMed] [Google Scholar]
- 24.Andersen LP, Kücükakin B, Werner MU, Rosenberg J, Gögenur I. Absence of analgesic effect of intravenous melatonin administration during daytime after laparoscopic cholecystectomy: a randomized trial. J Clin Anesth. 2014;26(7):545–550. doi: 10.1016/j.jclinane.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Gögenur I, Kücükakin B, Bisgaard T, et al. The effect of melatonin on sleep quality after laparoscopic cholecystectomy: a randomized, placebo-controlled trial. Anesth Analg. 2009;108(4):1152–1156. doi: 10.1213/ane.0b013e31819a6cf0 [DOI] [PubMed] [Google Scholar]
- 26.Madsen MT, Hansen MV, Andersen LT, et al. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. JCSM. 2016;12(2):225–233. doi: 10.5664/jcsm.5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen MV, Madsen MT, Andersen LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Int J Breast Cancer. 2014;2014:416531. doi: 10.1155/2014/416531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 30.Vij V, Dahiya D, Kaman L, Behera A. Efficacy of melatonin on sleep quality after laparoscopic cholecystectomy. Indian J Pharmacol. 2018;50(5):236–241. doi: 10.4103/ijp.IJP_250_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarkandi A, Naguib M, Riad W, et al. Melatonin vs. midazolam premedication in children: a double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005;22(3):189–196. doi: 10.1097/00003643-200503000-00005 [DOI] [PubMed] [Google Scholar]
- 32.Seet E, Liaw CM, Tay S, Su C. Melatonin premedication versus placebo in wisdom teeth extraction: a randomised controlled trial. Singapore Med J. 2015;56(12):666–671. doi: 10.11622/smedj.2015186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Melatonin secretion after surgery. Lancet. 2000;356(9237):1244–1245. doi: 10.1016/S0140-6736(00)02795-1 [DOI] [PubMed] [Google Scholar]
- 34.Kärkelä J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46(1):30–36. doi: 10.1034/j.1399-6576.2002.460106.x [DOI] [PubMed] [Google Scholar]
- 35.Li T, Jiang S, Han M, et al. Exogenous melatonin as a treatment for secondary sleep disorders: a systematic review and meta-analysis. Front Neuroendocrinol. 2019;52:22–28. doi: 10.1016/j.yfrne.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 36.Su X, Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anaesthesiol. 2018;31(1):83–88. doi: 10.1097/ACO.0000000000000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 38.Chinoy ED, Cuellar JA, Huwa KE, et al. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep. 2021;44(5). doi: 10.1093/sleep/zsaa291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin D, Huang X, Sun Y, Wei C, Wu A. Perioperative sleep disorder: a review. Front Med. 2021;8:640416. doi: 10.3389/fmed.2021.640416 [DOI] [PMC free article] [PubMed] [Google Scholar]