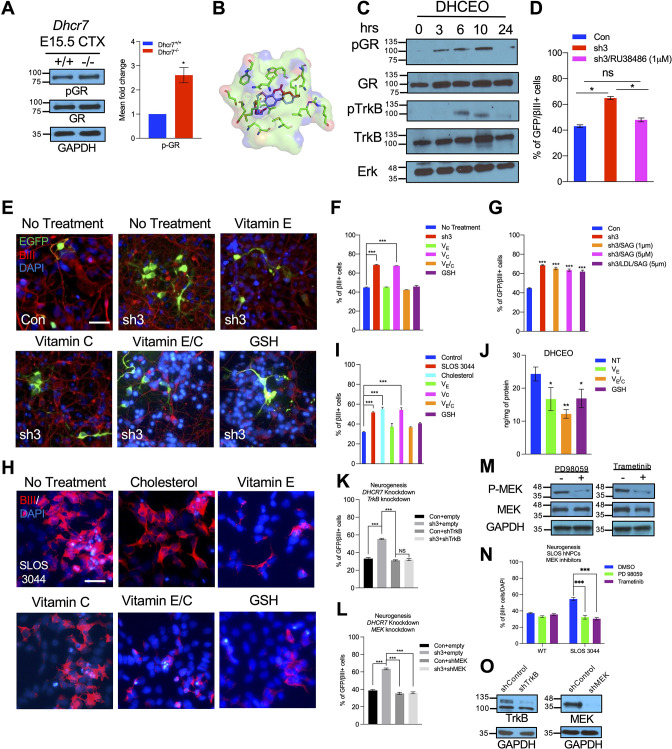
Figure 8. DHCEO activates cortical neurogenesis via activation of glucocorticoid receptor and inhibition of the effect or the formation of DHCEO rescues the neurogenic defects in SLOS NPCs.
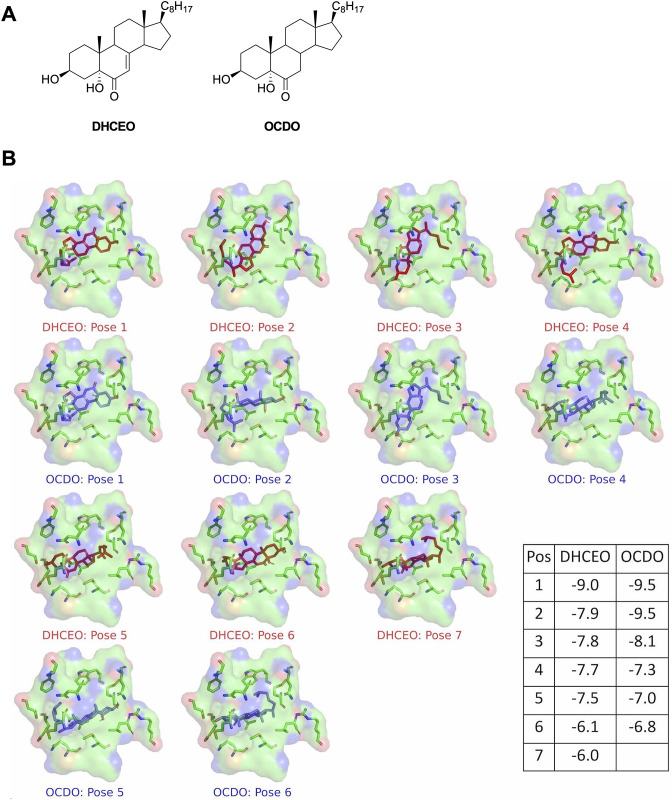
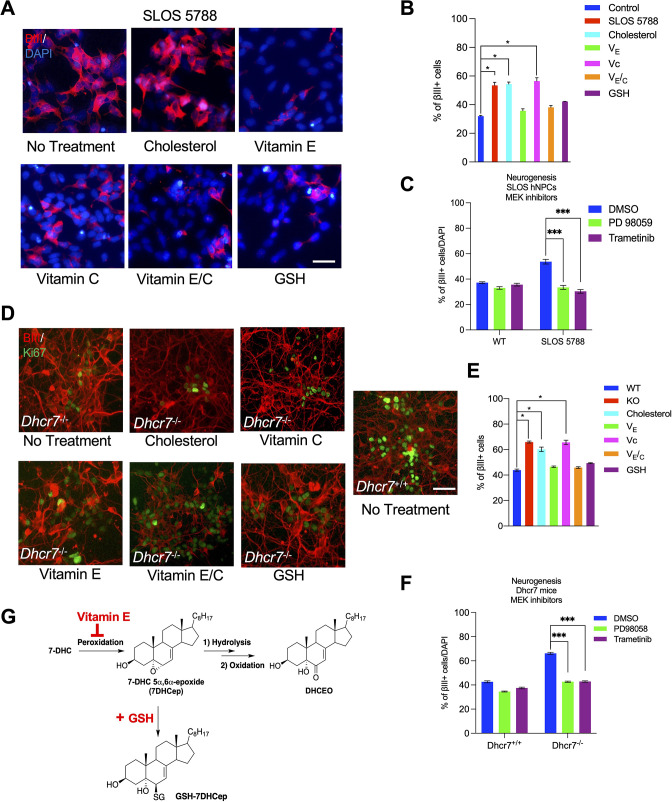
(A) Western blot showing increased phospho-GR in E15.5 Dhcr7-/- mouse brain relative to Dhcr7+/+. (B) Image of the docked position of DHCEO (red) and OCDO (blue) in the ligand binding pocket of GR. (C) Human neural progenitors were treated with 3.5 μM DHCEO over the indicated time periods. Lysates were probed with phosphor-GR and phosphor-TrkB and re-probed with antibodies for total GR, total TrkB or total ERK as loading controls. (D) Human NPCs were transfected with EGFP-control (Con) or EGFP-DHCR7 (sh3) shRNA. Cells were treated with 1 μM RU38486, a selective GR antagonist 1 day after transfection. Three days post-transfection, cells were immunostained for EGFP and βIII-tubulin and quantified. (E) Control hNPCs were transfected with EGFP-control (Con) or EGFP-DHCR7 shRNA (sh3), and then treated with vitamin E, vitamin C, vitamin E/C, or glutathione (GSH). Three days post-transfection, cells were immunostained for EGFP and βIII-tubulin. (F,G) Quantification of EGFP and βIII-tubulin + cells in DHCR7-KD Control hNPCs treated with various antioxidants, SAG, or LDL + SAG. (H) SLOS hNPCs were treated with cholesterol or various antioxidants and were immunostained for βIII-tubulin and DAPI. (I) Quantification of the proportion of βIII-tubulin + cells in Control hNPCs, and SLOS hNPCs treated with cholesterol (LDL) or various antioxidants. (J) Quantification of DHCEO by LC-MS/MS in SLOS hiPSCs treated with vitamin E, vitamin E/C, and GSH. (K, L) hNPCs were transfected with EGFP-control (Con) or EGFP-DHCR7 and co-transfected with TrkB shRNA (K) or MEK shRNA (L) vector. Three days later, cultures were immunostained for EGFP and βIII-tubulin and the proportion of transfected newborn neurons was determined. (M) hNPCs were treated or not treated with MEK/ERK inhibitors, trametinib or PD98059. Western blot of phosphor-MEK. The blots were then re-probed with antibodies for total MEK as loading controls. (N) SLOS hNPCs were treated with vehicle control or MEK/ERK inhibitors, trametinib (100 nM; purple column) or PD98059 (50 μM; green column). Three days later, cultures were immunostained for βIII-tubulin, and the proportion of new neurons was determined. (O) Western blots for TrkB or MEK1/2 in the lysate of 293T cells transfected with control or TrkB shRNA or MEK shRNA vector. The blots were re-probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Error bars indicate SEM. *, p<0.05; **, p<0.005; ***, p<0.001. n=3 per experiment. Scale Bar = 50 μm.