Abstract
Background and Objectives
Hereditary spastic paraplegia (HSP) causes progressive spasticity and weakness of the lower limbs. As neurologic examination and the clinical Spastic Paraplegia Rating Scale (SPRS) are subject to potential patient-dependent and clinician-dependent bias, instrumented gait analysis bears the potential to objectively quantify impaired gait. The aim of this study was to investigate gait cyclicity parameters by application of a mobile gait analysis system in a cross-sectional cohort of patients with HSP and a longitudinal fast progressing subcohort.
Methods
Using wearable sensors attached to the shoes, patients with HSP and controls performed a 4 × 10 m walking test during regular visits in 3 outpatient centers. Patients were also rated according to the SPRS, and in a subset, questionnaires on quality of life and fear of falling were obtained. An unsupervised segmentation algorithm was used to extract stride parameters and respective coefficients of variation.
Results
Mobile gait analysis was performed in a total of 112 ambulatory patients with HSP and 112 age-matched and sex-matched controls. Although swing time was unchanged compared with controls, there were significant increases in the duration of the total stride phase and the duration of the stance phase, both regarding absolute values and coefficients of variation values. Although stride parameters did not correlate with age, weight, or height of the patients, there were significant associations of absolute stride parameters with single SPRS items reflecting impaired mobility (|r| > 0.50), with patients' quality of life (|r| > 0.44), and notably with disease duration (|r| > 0.27). Sensor-derived coefficients of variation, on the other hand, were associated with patient-reported fear of falling (|r| > 0.41) and cognitive impairment (|r| > 0.40). In a small 1-year follow-up analysis of patients with complicated HSP and fast progression, the absolute values of mobile gait parameters had significantly worsened compared with baseline.
Discussion
The presented wearable sensor system provides parameters of stride characteristics which seem clinically valid to reflect gait impairment in HSP. Owing to the feasibility regarding time, space, and costs, this study forms the basis for larger scale longitudinal and interventional studies in HSP.
Hereditary spastic paraplegias (HSPs) are a heterogeneous group of rare genetic diseases characterized by progressive degeneration of the corticospinal tract.1,2 Age at onset and rate of progression of HSP show a wide variability, which is in part attributed to the underlying genetics.3 However, the clinical hallmark across all genetic subtypes and distinct clinical phenotypes is a progressive lower limb spasticity and weakness, leading to an insidiously worsening spastic gait disorder and—eventually—to loss of ambulation in many cases.4,5-6 In conjunction with reduced sensory input and loss of bladder control, this constitutes the clinical hallmark of “pure HSP.”4
No disease modifying or causal therapies are available for HSP yet, and objective biomarkers to measure disease progression are sparse. Although clinical examination and a rating scale are established to quantify impaired gait and mobility in HSP, these scales are subject to potential patient-dependent and clinician-dependent variance. Different stationary gait analysis systems have been applied in HSP.7-10 Although these studies mostly focused on the identification of specific movement patterns using optical motion capture systems, their use is limited in particular by space requirements, long setup and measurement times, the need for specialized staff, and high equipment costs. We have previously validated the technical calculation of gait cycle parameters in patients with HSP using a mobile gait analysis system based on an inertial measurement unit fixed to the patient's shoes.11,12 However, a detailed understanding of the feasibility and the clinical potential of the extracted gait parameters in a larger multicenter cohort of patients with HSP has been lacking.
Applying this mobile instrumented gait analysis system in clinical practice at 3 academic movement disorder centers, the aims of this study were (1) to compare gait cycle parameters between HSP and matched controls, (2) to investigate the relation of these gait parameters to clinical and patient-reported measures of impaired gait in HSP, and (3) to analyze longitudinal changes of gait parameters after 1 year.
Methods
Recruitment
Patients were recruited during routine outpatient visits within the HSP outpatient units of the Department of Molecular Neurology at the University Hospital Erlangen (n = 70 patients and 63 controls), the Department of Neurology at the University Hospital Essen (n = 29 patients and 46 controls), and the Center of Neurology at the University Hospital Tübingen (n = 13 patients and 3 controls), within the framework of a German clinical consortium (TreatHSP.net). Inclusion criteria were a genetic or clinical diagnosis of HSP and the ability to walk over a distance of at least 10 m with or without the use of a walking aid. Controls were recruited from accompanying spouses and relatives in whom familial HSP variants had been excluded and who showed no neurologic or other disease affecting gait. Additional controls were recruited from the local general population. As there are no major differences between the clinical phenotypes of genetic, familial, and sporadic cases and their symptomatic treatment, HSP patients with a clinical diagnosis, but without a genetic diagnosis were also included in this study (Figure 1C).
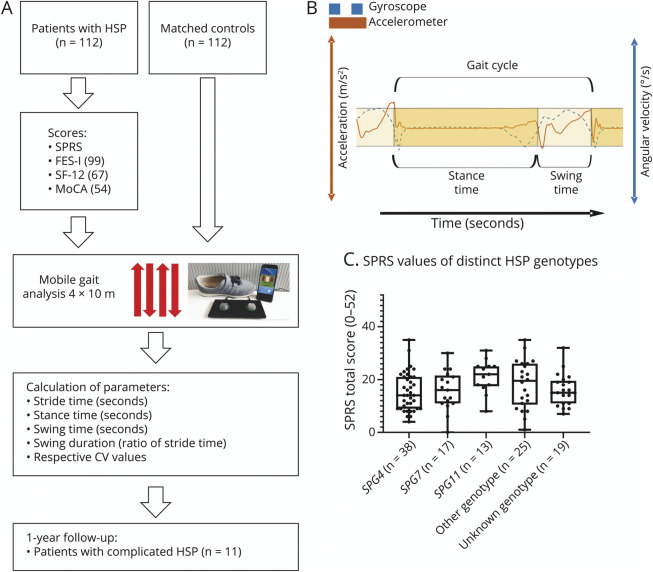
Figure 1. Study Design and Cohort.
(A) Cross-sectional study design: Patients with HSP from 3 academic centers and matched controls underwent clinical examination and instrumented mobile gait analysis performing the standardized 4 × 10 m walking test. Gait cyclicity measures were extracted using HSP-specific pattern recognition algorithms. A longitudinal gait analysis was performed in a subcohort with fast disease progression. (B) Schematic illustration of the temporal and spatial extraction of gait parameters from inertial measurement unit signals. (C) Depiction of scores on the SPRS scale (scale ranges from 0 to 52) within the different HSP genotypes. CV = coefficient of variation; HSP = hereditary spastic paraplegia; SPRS = Spastic Paraplegia Rating Scale.
Procedure/Design
The study design is depicted in Figure 1A. Patients with HSP were rated based on the Spastic Paraplegia Rating Scale (SPRS, with scores ranging from 0 to 52 and higher scores indicating more severe disease13). For instrumented gait analysis, patients and controls performed a 4 × 10 m walking test while wearing inertial measurement units fixed to the patients' shoes at the respective outpatient units.14 Gait cyclicity parameters were calculated from the sensor signals applying a previously established algorithm based on Hidden Markov models.11 Hereby, single steps are correctly segmented despite missing heel strike and despite continuous foot movement even during the stance phase in HSP gait, as previously described in the technical validation study.12 All patients and controls also performed the timed up and go test (TUG).15 Subsets of patients rated their perceived tendency to fall using the Falls Efficacy Scale International questionnaire (FES-I16; n = 99) and quality of life using the Short Form 12 (SF-1217; n = 67) questionnaire. In a minor subset of patients (n = 54), the Montreal Cognitive Assessment (MoCA)18 test was performed to assess cognitive deficits. Application of these questionnaires and tests was added to the study protocol after recruitment had already started.
For the longitudinal analysis of gait parameters, an additional gait analysis was performed in 11 patients with complicated HSP due to the anticipated rapid deterioration and consequent increase in disability. The follow-up interval ranged from 9 to 21 months, with a mean of 14 months.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants provided written informed consent as approved by the respective local ethics committees: Friedrich-Alexander-Universität Erlangen-Nürnberg (No. 166_18B and 347_17B), University of Essen (No. 19-8762-BO), and University of Tübingen (No. 347/2019BO2).
Gait Analysis
Patients were instructed to use their walking aid if regularly used for a distance of 40 m. To validate step recognition,12 the gait task was videotaped in a subset of patients. The Mobile GaitLab system consists of 2 inertial measurement units which are rigidly attached to shoes.19 Gyroscopic and accelerometric measures are transmitted to a mobile device during the gait tests. On the respective outpatient unit hallways of sufficient width, patients and controls were asked to walk over an outlined distance of 4 × 10 m as fast as possible without increasing risk of falling.
Stride Segmentation and Extraction of Gait Cycle Measures
A previously developed and technically validated algorithm was used for segmentation of gait data into stance and swing phases.12 It uses gyroscopic data from the sagittal plane and the frontal plane accelerometer to calculate specific signal features. Consequently, a hidden Markov model separates the recorded inertial measurement unit data into swing and stance phases. The algorithm was personalized for each patient and recording session within this study before the calculation of gait parameters. In addition, the visual results were postprocessed manually to exclude turning strides from the analysis. Furthermore, single swing phases with no preceding stance phase were manually deleted.
Sensor signals from each individual's 4 × 10 m walking test were segmented into stance phase and swing phase. Since most patients showed neither self-rated nor clinical differences between both legs, steps recorded from the left foot and the right foot were both included in the identical analysis. Both the initiation and the termination step of each of the 4 bouts showed a considerable variability compared with the remaining steps. Initiation and termination steps of each bout were therefore not included in the analysis of the 4 calculated gait cycle parameters and their respective coefficient of variation (CV) values because this yielded increased correlation with total SPRS values, as determined by Spearman ρ values. Two patients aborted the 4 × 10 m walk test after 1 × 10 m because of exhaustion, and these recordings were also included in the analyses.
Statistical Analysis
The Mann-Whitney U test was used to evaluate baseline differences between matched groups in nonparametric or nonnormally distributed parameters. Longitudinal data were compared with baseline data using the Wilcoxon matched-pairs signed rank test. Correlation analysis between functional gait measures and clinical rating scores or self-rating questionnaires was performed using Spearman rank correlation (ρ). p Values for multiple correlations reported in eTable 1 (links.lww.com/WNL/C118) were adjusted applying the Holm-Sidak method at α = 0.05. All statistical analyses were performed using SPSS software package version 21 (released 2012; IBM Corp., Armonk, NY), and graphs were prepared using Prism 8.3.0 (GraphPad, San Diego, CA).
Data Availability
The data sets used and/or analyzed during this study are available from the corresponding authors on request.
Results
Recruitment
A total of 112 ambulatory patients with HSP together with 112 control persons were enrolled at 3 academic HSP centers. There were no substantial differences of age, sex, height, and body weight between HSP and controls (Table 1). SPG4, SPG7, and SPG11 were the most frequently affected genes (34%, 17%, and 13%, respectively), which is in line with the previously reported proportional prevalence based on European HSP registries.3,20 No confirmed genetic diagnosis had been established in 19 patients (17%), but they fulfilled clinical diagnostic criteria for spastic paraplegia. Patients with HSP at all disease stages (if ambulatory over at least 10 m) were included, whereby SPRS scores ranged from 1 to 35 (mean total SPRS score of 16.8; Figure 1C; Table 1).
Table 1.
Patient Characteristics
Specific Gait Cycle Alterations in HSP
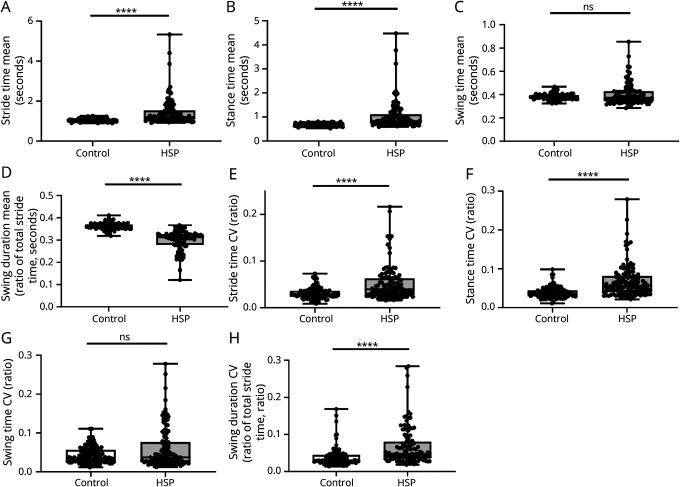
In HSP, there was a significant increase in stride time by 13% (Figure 2A), which seemed more pronounced for a subset of patients. Dissecting stride times into stance time and swing time compared with controls, we observed a significant increase in stance times by 22% (Figure 2B), but not in swing times (Figure 2C). In fact, absolute swing time seemed increased in a subset of patients, but decreased in the majority of patients. To normalize stance time and swing time to total stride times, we calculated swing duration, that is, a ratio of swing time to total stride time per gait cycle. There was a significant reduction of swing duration by 14% in HSP compared with controls (Figure 2D).
Figure 2. Mobile Gait Parameters in Patients With HSP and Controls.
Differences in mobile gait parameters comparing HSP and controls. (A–D) Absolute values for (A) stride time, (B) stance time, (C) swing time, and (D) swing duration (ratio of total stride time). (E–H) In addition, respective CVs were compared between controls and HSP. n = 112 for controls and HSP; ****p < 0.0001 according to the unpaired Mann-Whitney U test. CV = coefficients of variation; HSP = hereditary spastic paraplegia; ns = not significant; SPRS = Spastic Paraplegia Rating Scale.
Increased Gait Variability in HSP
Comparing patients with HSP with controls, CV values were significantly increased for both stride time by 36% (Figure 2E) and stance time by 55% (Figure 2F). As observed for the absolute values of swing time (Figure 2C), there was no change of swing time CV values in HSP (Figure 2G). However, the CV values of relative swing duration (normalized to total stride time) were significantly increased by 59% in HSP (Figure 2H).
Gait Parameters Correlate With Disease Severity
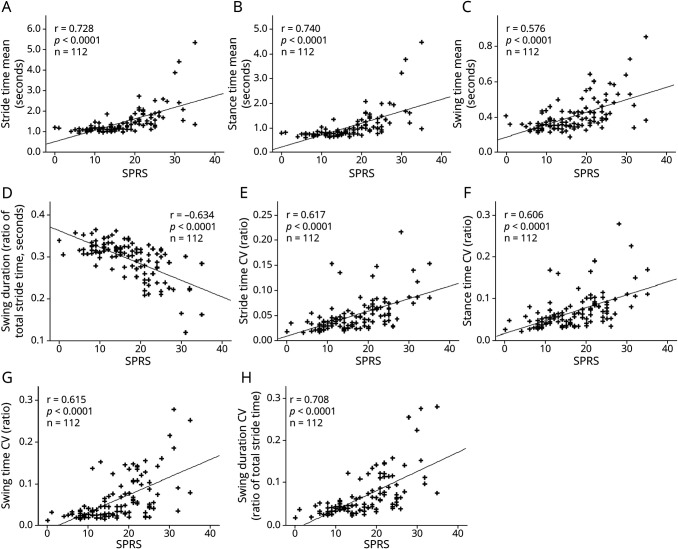
According to nonparametric Spearman ρ correlation analyses, significant correlations between SPRS values and all 4 absolute gait cycle measures were observed (Figure 3, A–D). For the parameters presented, ρ correlation coefficients were >0.5, indicating intermediate effect sizes. The total SPRS scores correlated with the 4 respective CV values of each gait cycle parameter showing an intermediate effect size and a significance level of similar range (Figure 3, E–H).
Figure 3. Correlation of Sensor-Based Gait Parameters With Total SPRS Values.
Correlation of mobile gait parameters with SPRS values, showing significant correlations with (A) stride time, (B) stance time, (C) swing time, (D) swing duration (ratio of stride time), and the respective CV values (E–H). CV = coefficient of variation; HSP = hereditary spastic paraplegia; r = Spearman ρ; SPRS = Spastic Paraplegia Rating Scale.
Gait Cycle Measures Reflect Functional Aspects in HSP
We next sought to analyze which aspects of disease severity (as reflected by the SPRS score) were specifically associated with alterations in gait cycle parameters.13 According to multiple correlation analyses as presented in Figure 4A and eTable 1 (links.lww.com/WNL/C118), all individual values of the “functional mobility” SPRS items 1–6 were significantly associated with both, absolute and CV gait cycle parameters, showing an intermediate effect size (|r| > 0.50). The clinical motor ratings for spasticity, weakness, and contractures (7–11) were also significantly associated with most gait cycle parameters, however, at lower effect sizes (|r| > 0.22). Contractures are rated on subitem 11, and the majority of patients with HSP in our cohorts showed no contractures (51/112 patients) or merely a mild, nonfixed abnormal joint position (52/112 patients). The 2 last subitems, 12 as a measure of spastic paraplegia-related pain and 13 of bladder and bowel dysfunction, did not significantly correlate with gait cycle measures.
Figure 4. Correlation of Sensor-Based Gait Parameters With SPRS Subitems and Patient Characteristics.
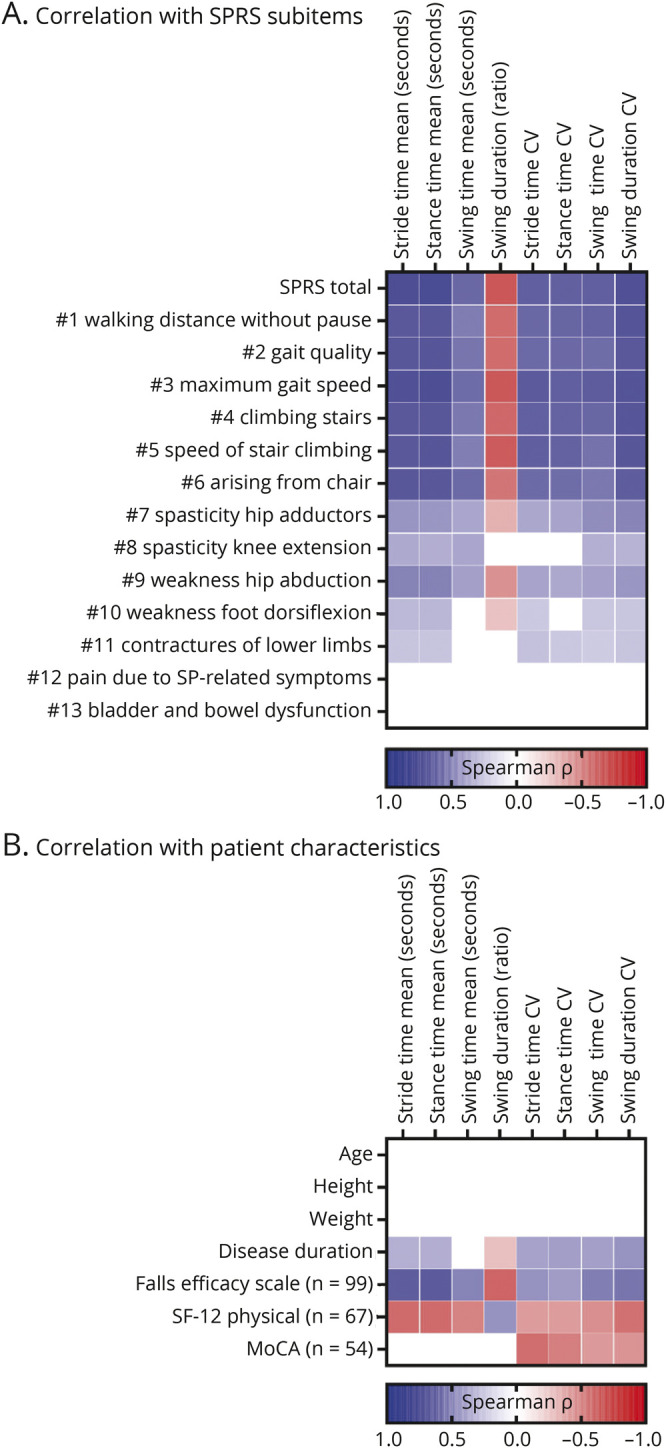
Heatmaps of correlation matrices demonstrating the associations of mobile gait parameters with (A) subitems of the SPRS scale and (B) patient characteristics. Spearman ρ is color coded from blue (p = +1.0) over white (p = 0) to red (p = −1.0). Only significant correlations with p values <0.05 after adjusting for multiple testing using the Holm-Sidak method (α = 0.05) are indicated. n = 112 unless otherwise indicated. CV = coefficient of variation; HSP = hereditary spastic paraplegia; MoCA = Montreal Cognitive Assessment score; SF-12 = short form quality-of-life questionnaire; SP = spastic paraplegia; SPRS = Spastic Paraplegia Rating Scale.
Mobile Gait Analysis Measures Associate With Quality of Life and With Fear of Falling
There were no significant correlations of each of the absolute and CV parameters with age, height, or body weight (Figure 4B). Compatible with the association of mobile gait parameters with SPRS values and the different rates of disease progression in HSP, there was a fair correlation of most gait parameters with disease duration (|r| > 0.27). The FES-I questionnaire reflects patient-reported fear of falling and was available in 88% of the patients. FES-I scores (n = 99) showed significant correlations with gait parameters, specifically with stride time (r = 0.63) and stance time (r = 0.64), but also with their respective CV parameters (r > 0.41). It is important to note that there were moderate correlations of absolute and CV gait parameters with physical quality of life, as measured by the SF-12 questionnaire (|r| > 0.39; n = 67). Finally, cognitive function as evaluated by scores of the MoCA scale was significantly associated with CV values for all stride parameters (|r| > 0.40; n = 54), but not with the absolute values of these stride parameters.
Longitudinal Change of Mobile Gait Parameters in a Fast Progressing Subcohort
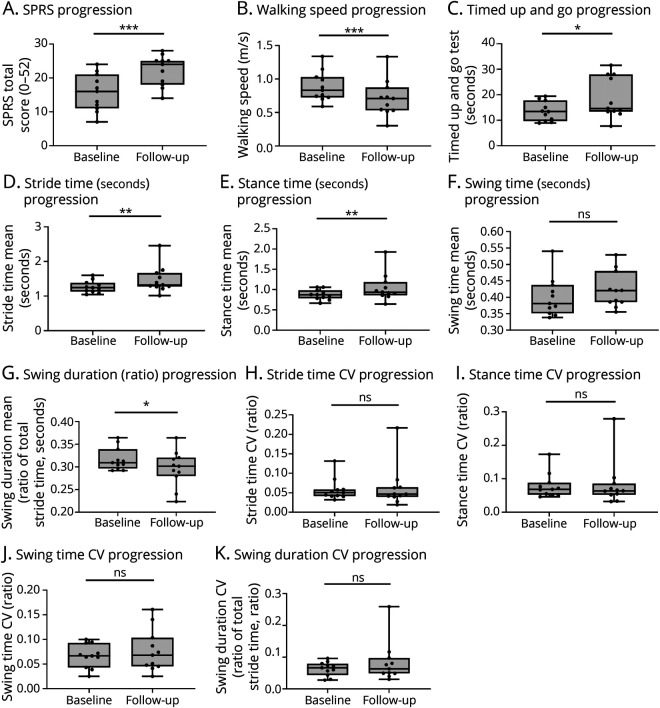
As a next step toward the validation of mobile gait parameters to capture impairment of gait in HSP, we performed a longitudinal follow-up gait analysis in a subcohort of 11 patients with HSP as a proof-of-principle. Owing to the high variability of progression in HSP, we selected patients with a fast progressing gait impairment. To this end, only patients with a complicated phenotype, that is, the presence of additional symptoms such as motor neuropathy or cognitive impairment, were included. After a mean interval of 14 months, there was a significant deterioration of SPRS scores, gait speed, and time to complete the TUG task (Figure 5, A–C). Comparing gait cycle parameters between baseline and follow-up, there was a significant increase in stride time and in stance time, and a significant decrease of relative swing duration (Figure 5, D–G; eTable 2, links.lww.com/WNL/C118). This small substudy suggests that, in particular, cyclicity parameters “worsened” over time in this fast progressing HSP cohort. It is of interest that there were no significant changes of the CV parameters at the follow-up (Figure 5, H–K).
Figure 5. Longitudinal Progression of Mobile Gait Parameters in a Subcohort.
Longitudinal progression of gait impairment and mobile gait parameters in a subset of patients with complicated, fast progressing HSP after 1 year. (A) SPRS values, (B) walking speed over 10 m, and (C) time to complete the timed up and go task at baseline and follow-up. (D–G) Absolute values for (D) stride time, (E) stance time, (F) swing time, and (G) swing duration (ratio of total stride time) at baseline and follow-up. (H–K) Respective CVs at baseline and follow-up. n = 11; ns = not significant; *p < 0.05, **p < 0.01, ***p < 0.001, according to the Wilcoxon signed rank test. CV = coefficient of variation; HSP = hereditary spastic paraplegia; SPRS = Spastic Paraplegia Rating Scale.
Discussion
The aim of this study was to investigate sensor-based gait assessment for HSP in a clinical setting by correlating sensor-based gait parameters with clinical scores in HSP. Focusing on temporal parameters of gait cycle components and their respective CV values, we observe significant changes compared with a control cohort matched for age and sex. Moreover, the present data demonstrate significant correlations with disease severity as reflected by total SPRS scores and specifically with gait-related quality of life. In addition, we demonstrate longitudinal changes of digital gait parameters with disease progression in a small subpopulation showing a rapid progression.
Our findings extend previous reports using 3-dimensional (3D) motion capture analyses. Using stationary approaches, altered ranges of motion in hip, knee, and ankle joints were observed for HSP when compared with controls and, in part, also with patients with cerebral palsy.7,8,21,22 In addition, 3D motion capture studies also characterized altered trunk movement patterns during gait in HSP.23,24 Moreover, different subtypes of gait impairments in HSP have been proposed, according to the severity and clinical phenotype of proximal vs distal spasticity and paresis of the legs.9,25,26 Although the presented mobile gait analysis may not be able to capture the entire spectrum of the trunk, pelvis, and lower extremities due to the restriction to 2 foot-worn sensors, our findings of increased stride time and stance time in HSP match previous reports in 3D motion capture analysis of HSP.27 We show that the proportional reduction of swing duration in HSP is not due to a consistent decrease in absolute swing time but rather due to the increase in stride times and specifically stance times. Prolongation of stance time may be the result of compensatory mechanisms to counteract postural instability or fear of falling and delayed toe/heel off due to spasticity or muscle weakness. As gait cyclicity measures intercorrelate with each other and we observed the highest Spearman ρ values for absolute stance time and stance time CV, it may be sufficient to focus on these 2 measures in future studies.
Compared with global measures of gait, such as gait speed on the 10-m walk test or distance on the 2-minute walk test, this study sets the ground to apply this mobile system also for long-term measurements, that is, outside of a clinical or gait laboratory setting even if annotation of mobile gait analysis data obtained during free living gait remains a challenge.19,28 Although the present mobile gait analysis system overcomes disadvantages associated with the use of stationary systems and is easily used by health care staff after a short training, segmentation of steps and gait bouts from processed data still requires manual curation by a trained computer scientist. Development of a graphical user interface is one of the crucial requirements to make this system applicable for clinical use in the future.
Disease duration was significantly correlated with both, absolute values of gait cycle measures and with their respective CV values. In conjunction with the significant associations of gait cycle measures with total SPRS values and SPRS items 1–6 reflecting functional gait impairment, our data suggest that the calculated gait cycle parameters also provide an objective and digital biomarker of disease progression in HSP. To this end, we provide a proof of principle in a small and defined subcohort of patients with HSP showing significant changes in specific gait cycle parameters after 1 year of follow-up. Additional longitudinal studies and larger patient cohorts are required to dissect the precise contribution of each of the presented parameters during progressive gait impairment.
SPRS items 7–11, reflecting spasticity and weakness of hip and ankle muscles as well as contractures, showed a lower association with gait cycle measures than the functional items 1–6 (Figure 4A). This may be due to a less homogeneous distribution of scores on these subitems in our HSP cohort; in addition, spasticity and weakness may influence gait in specific interactions rather than showing a linear correlation. Future machine learning-based development of additional algorithms and eventually introduction of additional mobile sensors may improve the prediction of these parameters and/or the disease course.
It is of interest that the absolute values of gait cycle components showed slightly higher associations with physical quality of life and patient-reported fear of falling than their respective variabilities (as measured by CV values, Figure 4B). These digital measures suggest that the physical component of quality of life and fear of falling are mainly affected by gait disability, that is, prolonged duration of gait cycle components and reduced ratio of the swing phase within the gait cycle.
Gait dysfunction is the major driver for impaired quality of life in HSP. In our cohort, gait cycle parameters were significantly correlated with physical quality of life, as measured by the SF-12 questionnaire (Figure 4B). While it has to be kept in mind that SF-12 was available for a subset of patients (67/112) solely, this matches our previous observation that SF-12 physical component summary scores correlate with SPRS, FES-I, and manually recorded gait measures.29 This is also in line with previous studies demonstrating (1) that the mental and physical component scores of the SF-36 questionnaire both correlated significantly with total SPRS values in a cohort of 143 German patients with HSP,30 (2) that health-related quality of life as measured by the RAND-36 questionnaire was associated with subjective changes in health in a cohort of 49 Estonian patients with HSP,31 (3) that spastic gait in HSP causes withdrawal from social activities and shame in 14 Dutch patients with HSP as determined by semistructured interviews,32,33 and (4) that specific interventions to improve mobility also lead to improved quality of life34 and fatigue.35,36 Moreover, the maximum gait distance correlated with quality of life as measured with the EuroQol-5 dimension questionnaire in 118 SPG4 patients.37 Thus, symptomatic treatment to improve gait and eventually also the prevention of progressive gait disability is an urgent need in clinical care and research for HSP. Our study paves the way for future application of mobile gait analysis in larger longitudinal studies, addressing either the natural history of gait impairment in HSP or the symptomatic effect of antispastic therapies such as baclofen or botulinum toxin.
Cognitive impairment, that is, a MoCA value below 26 points, was present in 31 of the 54 patients (57%). Of these, 23 patients were mildly cognitively impaired (MoCA 20–25), with 15 showing a pure phenotype of HSP. This is in line with previous observations that mild cognitive impairment is frequent even in pure types of HSPs, including SPG4, SPG5, and SPG7.37,38 A total of 8 patients showed a moderate to severe impairment (MoCA below 20 points), and 6 of these 8 patients had a complicated phenotype. Despite the limitation that only a subset of patients underwent the testing, cognitive impairment was significantly associated with increased gait variability, but not with absolute gait measures. This may be due to the high ratio of complicated HSP within the cognitively impaired patients. Alternatively, cognitive impairment itself might have led to an increased variability of gait during the applied 4 × 10 m paradigm. Indeed, it has been shown in the general population that both mild cognitive impairment and dementia increase gait variability more than gait speed, especially during gait paradigms applying a distraction task.39-41 Thus, it would be interesting to include an additional dual task gait test in future analyses of gait in HSP to test its effect on gait parameters in cognitively impaired vs unaffected patients.
Although this study demonstrates the relevance of mobile sensor-based gait cycle parameters in HSP, additional measures reflecting specific components of spastic gait in HSP might improve the prediction of SPRS values or the classification of different subtypes of spastic gait in HSP, as demonstrated before using motion capture analyses.9 Specifically, quantification of calf vs adductor muscle spasticity and paresis would be of clinical relevance, using parameters such as maximum toe clearance, heel strike angle, or gait width, respectively.42 To this end, further bioinformatic development of feature extraction from inertial measurements in HSP is needed in the future.11,12
Several limitations have to be kept in mind when interpreting the presented data. First, it is important to note that the correlative analyses of this study did not control for intercorrelations of different factors and that correlations do not imply causality. We also did not control for antispastic therapy, that is, physiotherapy; use of walking aids; current oral, intrathecal, or intramuscular medication; and previous surgery. Second, most of the observed correlations, with absolute values ranging from 0.2 to 0.7, are weak to moderate, which means that none of the mobile gait parameters perfectly matches with certain SPRS items. It is important that questionnaires and cognitive testing measures were obtained only in subsets of patients (FES-I: 88%; SF-12: 60%; MoCA: 48%). Although this was related to organizational circumstances rather than insufficient return of questionnaires, a certain degree of selection bias may have been introduced. In addition, the design of our longitudinal study, specifically focusing on a subcohort of HSP patients with a complicated phenotype, is subject to a selection bias. Owing to the small sample size of 11 patients, these data may not reflect the mean rate of progression in complicated HSP. Moreover, these data on disease progression cannot be extrapolated to patients with HSP in general. Finally, when interpreting CV values, it has to be kept in mind that they do not reflect gait variability in general but rather specifically indicate the variability of the respective gait cycle phase during the applied 4 × 10 m test. Nevertheless, the present results with its cross-sectional multicenter design in a relatively large patient cohort with a rare movement disorder and first longitudinal data provide a powerful and promising approach to objectively validate the application of mobile gait analysis in HSP.
Performed during specific gait tasks in a hospital setting, mobile gait analysis provides cyclicity parameters that correlate well with measures of disease severity, patient-reported outcomes, and gait impairment in HSP. This study builds the basis for future applications in monitoring disease progression, objective assessment of therapeutic intervention, and studying mobility in HSP outside of a gait laboratory environment.
Acknowledgment
The authors thank all patients for their participation in this study. Teresa Greinwalder and Katrin Dillmann provided excellent assistance in gait recordings and data storage. All members of the mobility laboratory, especially Kathrin Kinscher, Julia List, and Evelyn Loris, are gratefully acknowledged for performing gait recordings. The present work was performed in partial fulfillment of the requirements for obtaining the degree Dr. med. (I.T.S.).
Glossary
- 3D
3-dimensional
- CV
coefficient of variation
- FES-I
Falls Efficacy Scale International questionnaire
- HSP
hereditary spastic paraplegia
- MoCA
Montreal Cognitive Assessment
- SF-12
Short Form 12
- SPRS
Spastic Paraplegia Rating Scale
- TUG
timed up and go test
Appendix. Authors
Study Funding
This work was supported by the German Bundesministerium für Bildung und Forschung (BMBF) through the TreatHSP consortium (01GM1905B to M.R., J.W., and H.G., 01GM1905A to R.S., 01GM1905C to S.K.), by the Deutsche Forschungsgemeinschaft (German Research Foundation; 270949263/GRK2162 to M.R. and J.W.; Heisenberg professorship program grant 526 number ES 434/8-1 to B.E.), by the European Union's Horizon 2020 research and innovation program under the frame of the EJP-RD network PROSPAX (No 441409627 to R.S.), by the German Network of Neurodegenerative Disorders (DZNE), by the “Förderverein für HSP-Forschung,” and by the “Forschungsstiftung Medizin” at the University Hospital Erlangen. M.R. is a fellow of the Clinician Scientist Programme (IZKF, University Hospital Erlangen). R.S. is a member of the European Reference Network for Rare Neurological Diseases (ERN-RND). H.G. was supported by the Fraunhofer Internal Programs under Grant Nos. Attract 044-602140 and 044-602150.
Disclosure
M.O. reports—outside of the submitted work—part-time employment by Portabiles HealthCare Technologies GmbH which develops and marketizes the Mobile GaitLab system. B.E. holds a patent on human gait assessment and reports shares of Portabiles Healthcare Technologies GmbH. J.K. holds a patent on human gait assessment and reports shares of Portabiles Healthcare Technologies GmbH. J.W. holds a patent on human gait assessment (without holding shares). The other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Shribman S, Reid E, Crosby AH, Houlden H, Warner TT. Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019;18(12):1136-1146. [DOI] [PubMed] [Google Scholar]
- 2.Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174-183. [DOI] [PubMed] [Google Scholar]
- 3.Schüle R, Wiethoff S, Martus P, et al. Hereditary spastic paraplegia: clinicogenetic lessons from 608 patients. Ann Neurol. 2016;79(4):646-658. [DOI] [PubMed] [Google Scholar]
- 4.Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1(8334):1151-1155. [DOI] [PubMed] [Google Scholar]
- 5.Durr A, Brice A, Serdaru M, et al. The phenotype of “pure” autosomal dominant spastic paraplegia. Neurology. 1994;44(7):1274-1274. [DOI] [PubMed] [Google Scholar]
- 6.McDermott CJ, Burness CE, Kirby J, et al. Clinical features of hereditary spastic paraplegia due to spastin mutation. Neurology. 2006;67(1):45-51. [DOI] [PubMed] [Google Scholar]
- 7.Piccinini L, Cimolin V, D'Angelo MG, Turconi AC, Crivellini M, Galli M. 3D gait analysis in patients with hereditary spastic paraparesis and spastic diplegia: a kinematic, kinetic and EMG comparison. Eur J Paediatr Neurol. 2011;15(2):138-145. [DOI] [PubMed] [Google Scholar]
- 8.Wolf SI, Braatz F, Metaxiotis D, et al. Gait analysis may help to distinguish hereditary spastic paraplegia from cerebral palsy. Gait Posture. 2011;33(4):556-561. [DOI] [PubMed] [Google Scholar]
- 9.Serrao M, Rinaldi M, Ranavolo A, et al. Gait patterns in patients with hereditary spastic paraparesis. PLoS One. 2016;11(10):e0164623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Lith BJH, den Boer J, van de Warrenburg BPC, Weerdesteyn V, Geurts AC. Functional effects of botulinum toxin type A in the hip adductors and subsequent stretching in patients with hereditary spastic paraplegia. J Rehabil Med. 2019;51(6):434-441. [DOI] [PubMed] [Google Scholar]
- 11.Martindale CF, Roth N, Gasner H, Jensen D, Kohl Z, Eskofier BM. Mobile gait analysis using personalised hidden Markov models for hereditary spastic paraplegia patients. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:5430-5433. [DOI] [PubMed] [Google Scholar]
- 12.Martindale CF, Roth N, Ganer H, et al. Technical validation of an automated mobile gait analysis system for hereditary spastic paraplegia patients. IEEE J Biomed Health. 2019;24(5):1490-1499. [DOI] [PubMed] [Google Scholar]
- 13.Schüle R, Holland-Letz T, Klimpe S, et al. The Spastic Paraplegia Rating Scale (SPRS): a reliable and valid measure of disease severity. Neurology. 2006;67(3):430-434. [DOI] [PubMed] [Google Scholar]
- 14.Gassner H, Jensen D, Marxreiter F, et al. Gait variability as digital biomarker of disease severity in Huntington's disease. J Neurol. 2020;267(6):1594-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148. [DOI] [PubMed] [Google Scholar]
- 16.Dias N, Kempen GIJM, Todd CJ, et al. The German version of the Falls Efficacy Scale-International Version (FES-I) [in German]. Z Gerontol Geriatr. 2006;39(4):297-300. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. [DOI] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 19.Ullrich M, Hannink J, Gaßner H, Klucken J, Eskofier BM, Kluge F. Unsupervised harmonic frequency-based gait sequence detection for Parkinson's disease. 2019 IEEE EMBS Int Conf Biomed Heal Informatics (BHI). 2019:1-4. [Google Scholar]
- 20.Tesson C, Koht J, Stevanin G. Delving into the complexity of hereditary spastic paraplegias: how unexpected phenotypes and inheritance modes are revolutionizing their nosology. Hum Genet. 2015;134(6):511-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klebe S, Stolze H, Kopper F, et al. Gait analysis of sporadic and hereditary spastic paraplegia. J Neurol. 2004;251(5):571-578. [DOI] [PubMed] [Google Scholar]
- 22.Cimolin V, Piccinini L, D'Angelo MG, et al. Are patients with hereditary spastic paraplegia different from patients with spastic diplegia during walking? Gait evaluation using 3D gait analysis. Funct Neurol. 2007;22(1):23-28. [PubMed] [Google Scholar]
- 23.Bonnefoy-Mazure A, Turcot K, Kaelin A, Coulon GD, Armand S. Full body gait analysis may improve diagnostic discrimination between hereditary spastic paraplegia and spastic diplegia: a preliminary study. Res Dev Disabil. 2013;34(1):495-504. [DOI] [PubMed] [Google Scholar]
- 24.Adair B, Rodda J, McGinley JL, Graham HK, Morris ME. Kinematic gait deficits at the trunk and pelvis: characteristic features in children with hereditary spastic paraplegia. Dev Med Child Neurol. 2016;58(8):829-835. [DOI] [PubMed] [Google Scholar]
- 25.Serrao M, Chini G, Bergantino M, et al. Identification of specific gait patterns in patients with cerebellar ataxia, spastic paraplegia, and Parkinson's disease: a non-hierarchical cluster analysis. Hum Mov Sci. 2018;57:267-279. [DOI] [PubMed] [Google Scholar]
- 26.Pulido-Valdeolivas I, Gómez-Andrés D, Martín-Gonzalo JA, et al. Gait phenotypes in paediatric hereditary spastic paraplegia revealed by dynamic time warping analysis and random forests. PLoS One. 2018;13(3):e0192345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vugt Y, Stinear J, Davies TC, Zhang Y. Postural stability during gait for adults with hereditary spastic paraparesis. J Biomech. 2019;88:12-17. [DOI] [PubMed] [Google Scholar]
- 28.Gaßner H, Sanders P, Dietrich A, et al. Clinical relevance of standardized mobile gait tests. Reliability analysis between gait recordings at hospital and home in Parkinson's disease: a pilot study. J Parkinsons Dis. 2020;10(4):1763-1773. [DOI] [PubMed] [Google Scholar]
- 29.Gaßner H, List J, Martindale CF, et al. Functional gait measures correlate to fear of falling, and quality of life in patients with Hereditary Spastic Paraplegia: a cross-sectional study. Clin Neurol Neurosurg. 2021;209:106888. [DOI] [PubMed] [Google Scholar]
- 30.Klimpe S, Schüle R, Kassubek J, et al. Disease severity affects quality of life of hereditary spastic paraplegia patients. Eur J Neurol. 2011;19(1):168-171. [DOI] [PubMed] [Google Scholar]
- 31.Braschinsky M, Rannikmäe K, Krikmann Ü, et al. Health-related quality of life in patients with hereditary spastic paraplegia in Estonia. Spinal Cord. 2011;49(2):175-181. [DOI] [PubMed] [Google Scholar]
- 32.Kerstens HCJW, Satink T, Nijkrake MJ, et al. Stumbling, struggling, and shame due to spasticity: a qualitative study of adult persons with hereditary spastic paraplegia. Disabil Rehabil. 2019;42(26):1-8. [DOI] [PubMed] [Google Scholar]
- 33.van Lith BJH, Kerstens HCJW, van den Bemd LAC, et al. Experienced complaints, activity limitations and loss of motor capacities in patients with pure hereditary spastic paraplegia: a web-based survey in the Netherlands. Orphanet J Rare Dis. 2020;15(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertolucci F, Martino SD, Orsucci D, et al. Robotic gait training improves motor skills and quality of life in hereditary spastic paraplegia. Neurorehabilitation. 2015;36(1):93-99. [DOI] [PubMed] [Google Scholar]
- 35.Servelhere KR, Faber I, Martinez A, et al. Botulinum toxin for hereditary spastic paraplegia: effects on motor and non-motor manifestations. Arq Neuropsiquiatr. 2018;76(3):183-188. [DOI] [PubMed] [Google Scholar]
- 36.de Lima FD, Faber I, Servelhere KR, et al. Randomized trial of botulinum toxin type A in hereditary spastic paraplegia—the SPASTOX trial. Mov Disord. 2021;36(7):1654-1663. [DOI] [PubMed] [Google Scholar]
- 37.Rattay TW, Boldt A, Völker M, et al. Non-motor symptoms are relevant and possibly treatable in hereditary spastic paraplegia type 4 (SPG4). J Neurol. 2019;267(2):369-379. [DOI] [PubMed] [Google Scholar]
- 38.Jacinto-Scudeiro LA, Machado GD, Ayres A, et al. Are cognitive changes in hereditary spastic paraplegias restricted to complicated forms? Front Neurol. 2019;10:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual‐tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21(7):950-957. [DOI] [PubMed] [Google Scholar]
- 40.Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol Ser. 2008;63(12):1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control. 2012;16(1):64-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibrahim AA, Küderle A, Gaßner H, Klucken J, Eskofier BM, Kluge F. Inertial sensor-based gait parameters reflect patient-reported fatigue in multiple sclerosis. J Neuroeng Rehabil. 2020;17(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during this study are available from the corresponding authors on request.