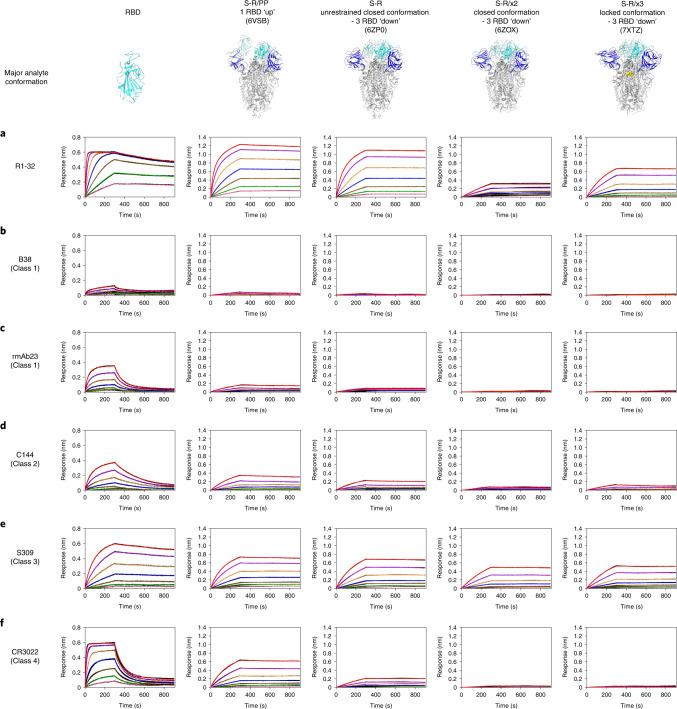
Fig. 3. Binding of R1-32 and selected antibodies of different RBD antibody classes to SARS-CoV-2 RBD and spikes in different conformations.
a–f, Binding of R1-32 (a), B38 (ref. 59), (b), rmAb23 (ref. 23), (c), C144 (ref. 2), (d), S309 (ref. 60) (e) and CR3022 (ref. 61) (f) from different RBD-targeting antibody classes (see Extended Data Fig. 7 and Supplementary Fig. 3 for their classification and epitopes) to SARS-CoV-2 RBD and spike trimers of different conformations. Characterized previously, major conformations of S-R/PP, S-R, S-R/x2 and S-R/x3 spikes used as analytes in the BLI assays are shown in the top row. S-R/PP (2P-stabilized spike with furin site changed to a single R) and S-R (unstabilized native spike with furin site changed to a single R) have been shown to exist primarily in 1 RBD ‘up’ (80% 1 RBD ‘up’, 20% closed) and unrestrained closed (20% 1 RBD ‘up’, 80% closed) conformations, respectively8. RBDs in S-R/x2 and S-R/x3 spikes were restrained by the x2 and x3 disulfide bonds in ‘down’ positions, and the two spikes differ slightly in trimer packing, adopting primarily closed8 and locked42 conformations, respectively. IgGs were immobilized onto Protein A biosensors and submerged into 2-fold serially diluted RBD and spike solutions (200, 100, 50, 25, 12.5, 6.25, 3.125 nM) to record sensorgrams (black lines). The fits of the data are shown as coloured lines. Rate constants (kon and koff) estimated from the association and dissociation phases and dissociation constants (KD) derived from kinetic analyses are summarized in Supplementary Table 3.