Summary
The murine collagen antibody-induced arthritis (CAIA) model resembles various features of human rheumatoid arthritis and is based on the intraperitoneal or intravenous injection of autoantibodies against type II collagen. Here, we present a standardized protocol for the intraperitoneal injection of arthritis-inducing autoantibodies in mice, followed by a description of daily arthritis assessments. We then detail the steps to harvest joint and bone tissues for histological, radiological, and molecular analyses. We highlight animal welfare and 3R considerations for experimental arthritis studies.
For complete details on the use and execution of this protocol, please refer to Maleitzke et al. (2021, 2022).
Subject areas: Health sciences, Immunology, Microscopy, Model organisms, Antibody
Graphical abstract

Highlights
-
•
Protocol for the intraperitoneal injection of arthritis-inducing autoantibodies in mice
-
•
Can be used to analyze both acute inflammatory and chronic resolution phases of arthritis
-
•
Robust and established outcome parameters ensuring reproducible results
-
•
Particular attention paid to animal welfare and 3R principles
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The murine collagen antibody-induced arthritis (CAIA) model resembles various features of human rheumatoid arthritis and is based on the intraperitoneal or intravenous injection of autoantibodies against type II collagen. Here, we present a standardized protocol for the intraperitoneal injection of arthritis-inducing autoantibodies in mice, followed by a description of daily arthritis assessments. We then detail the steps to harvest joint and bone tissues for histological, radiological, and molecular analyses. We highlight animal welfare and 3R considerations for experimental arthritis studies.
Before you begin
To study the effector phase of rheumatoid arthritis (RA), antibody-induced arthritis models are commonly employed. Following the binding of antibodies to respective antigens, complement activation and FcγR engagement facilitate arthritis onset and progression (Nandakumar and Holmdahl, 2006). Disease initiation in antibody-induced arthritis models occurs mainly independent of B and T cells, however immune cells still modulate the local and systemic inflammation present during the effector phase (Nandakumar et al., 2004).
While the K/BxN serum transfer model is based on autoantibodies against glucose-6-phosphate isomerase and allows studying the alternative complement activation, the collagen antibody-induced arthritis (CAIA) model relies on autoantibodies against collagen type II, and activates both, the classical and the alternative complement pathway (Nandakumar and Holmdahl, 2006). Furthermore, the CAIA model is independent of major histocompatibility complex (MHC) and commercially available antibody cocktails usually consist of four arthritis-inducing monoclonal antibodies, specific for epitopes located on type II collagen (C1, D3, J1, and U1 on CB8, CB10 and CB11 fragments). Once antibodies bind to epitopes, immune complex formation and deposition in cartilage and synovium cause a complement-dependent, inflammatory reaction. Immune complexes further activate monocytes and the release of pro-inflammatory cytokines, which recruit macrophages and neutrophils to the site of inflammation (Nandakumar et al., 2003). As T and B cell-deficient mice develop a milder form of arthritis, T and B cells seem to modulate arthritis severity at the effector level in this model, while disease initiation is independent of these cells. The CAIA model is a transient disease model where the acute inflammatory phase (up to day 14) is followed by gradually subsiding arthritis resembling the chronic resolution phase (up to day 48), where signs of inflammation and joint swelling slowly decrease (Matzelle et al., 2012; Nandakumar and Holmdahl, 2006).
Alternative murine arthritis models, which include spontaneous, active immunization, chemically-induced and humanized models have previously been reviewed in depth (Benson et al., 2018; Caplazi et al., 2015; Schinnerling et al., 2019).
This protocol describes the application of an antibody cocktail against type II collagen to induce CAIA in wildtype (WT) mice with a C57Bl/6J genetic background. CAIA can be induced in most mouse strains, including genetically modified animals, reaching arthritis incidences close to 100%. In previous studies we were able to examine the CAIA-joint phenotype in calcitonin gene-related peptide alpha-deficient (αCGRP−/−) (Maleitzke et al., 2021) and calcitonin receptor-deficient (Calcr−/−) mice (Maleitzke et al., 2022). Likewise, the model may also be used to evaluate treatment responses to specific pharmacologic agents in relation to placebo in WT or genetically modified mice. Standardized outcome measures can thus be assessed in almost any mouse strain, however dose titration of antibody cocktail may be required depending on the genetic background.
Institutional permissions
All experiments require ethical approval from the local animal welfare authorities and must be executed in compliance with the Animal Welfare Act (Federal Law Gazett I, p.1094) and the National Institutes of Health Guide for Care and Use of Laboratory Animals. For the underlying experiments of this protocol ethical approval (G-0044/18) was obtained from the local animal welfare organization (Landesamt für Gesundheit und Soziales in Berlin, Germany) and all experiments were carried out in accordance with institution guidelines.
Experimental concerns
-
1.
Mice should be housed in groups. Solitary housing is in most cases obsolete and should be refrained from when possible. Avoid changing housing and living conditions at any time to reduce external stress and outcome variability, without compromising hygiene standards.
-
2.
Animals should be allowed an accommodation of 2 weeks before experiment initiation. This should include daily handling by the same animal care takers and getting animals used to daily assessments of grip strength and ankle swelling measurements.
-
3.
Temperatures between 22°C–24°C and a 12 h light-dark cycle shall be ensured. Mice should be fed a standard diet with access to water ad libitum enriched with analgesic metamizole (1,350 mg kg−1body weight day−1). Metamizole does not interact with the clinical course of arthritis and can therefore be used as an effective analgesic (Ruiz-Perez et al., 2017).
-
4.
The CAIA model works best in adult male mice and exposure to estrogen (in female mice) seems to partially attenuate CAIA (Nandakumar et al., 2003). Mice should have a minimum age of 10–12 weeks.
Note: Prevent mice from exposure to environmental lipopolysaccharide (LPS) by only using autoclaved materials. Exposure to LPS prior to the experiment could result in tolerance which may affect arthritis severity (Chondrex, 2022).
Preparation of monoclonal antibody cocktail injection (day 0)
Timing: 30 min
-
5.
Thaw commercially available arthritis-inducing antibody cocktail on ice, which must be stored at −20°C (we use the ArthritioMab antibody cocktail for this protocol; MD Bioproducts, Oakdale, MN, USA) (Figure 1).
Note: Avoid redundant freeze–thaw cycles to maintain antibody quality.
-
6.
Make sure mice are in good health and clean work surface with 70% ethanol.
-
7.Assemble required number of syringes and needles (27–30G), according to animal numbers (Figure 1).
-
a.Once the antibody cocktail has reached room temperature (20°C–24°C), draw up 8 mg (=400 μL) of ArthritioMab antibody cocktail (20 mg/mL) (MD Bioproducts) per animal.
-
a.
-
8.
Prepare an empty cage to safely house mice following injection (Figure 1).
Note: Dependent on the mouse strain/genetic background of animals and CAIA cocktail dose applied, arthritis severity may vary. Titration experiments with a small number of animals are therefore usually required. In a study comparing WT and CIKS-deficient animals (both with a C57Bl/6 background), authors used 4 mg of CAIA cocktail i.v. (Pisitkun et al., 2010). Other authors found that in the CD1 mouse strain, 8 mg of CAIA cocktail i.p. caused arthritis to develop and subside more rapidly than 3 mg applied to the same genetic strain (Tu et al., 2016). In our previous experiments, we found 8 mg of CAIA cocktail i.p. to be the ideal dose to study a full arthritic phenotype in animals with a C57Bl/6J genetic background (Maleitzke et al., 2021, 2022).
Optional: Besides the antibody cocktail from MD Bioproducts, alternative antibody cocktails are also commercially available, e.g., from Chondrex LLC, Woodinville, WA, USA (cat#53010; 53040, 53100), etc.
Note: To ensure adequate pain relief for all mice a minimum concentration of 1350 mg metamizole kg−1body weight day−1 is necessary. Average mice weigh between 20–30 g and consume 10 mL of water per day. Therefore, it is required to enrich drinking water with 4 mg/mL of metamizole. Instead of metamizole, 0.1 mg buprenorphine kg−1 body weight (50 μL) may be used subcutaneously as a potent analgesic alternative (Khachigian, 2006).
Note: To facilitate nesting and protect physical comfort following arthritis induction, SAFE® compact crinklets (Safe Diets, Rosenberg, Germany) or alternative environmental enrichments and soft bedding should be provided in each cage.
Figure 1.
Set-up before experiment initiation
Syringes and needles should be provided according to the number of animals to be injected. Ice is used to thaw the CAIA antibody cocktail. Alcohol is used to disinfect surfaces and a spare cage is required as a recovery cage following injections. A standard cage for experimental animals should be equipped with nesting material, food and drinking water enriched with analgesic metamizole.
Preparation of LPS injection (day 3)
Timing: 30 min
-
9.Mix 5 mg of LPS powder (Escherichia Coli serotype 055:B5) (MD Bioproducts) in 1 mL of phosphate buffered saline (PBS) in a sterile setting to obtain a 5 mg/mL solution.
-
a.Vortex shortly until all LPS is dissolved.
-
b.Fill a sterile 10 mL glass vial with 8 mL of PBS and add reconstituted LPS (1 mL).
-
c.Wash out LPS vial with 1 mL of PBS and add to the 10 mL glass vial to get 10 mL of 0.5 mg/mL LPS.
-
a.
Pause point: Can be stored at −20°C for extended periods.
Note: Use 200 μL of the final solution (100 μg LPS) per animal. The use of LPS significantly reduces the amount of antibodies required. However, adapted protocols without the use of LPS have also been described (MDBioproducts, 2022).
CRITICAL: Make sure to use sterile PBS for each step.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Metamizole | MSD Animal Health | Vetalgin 500 mg/mL |
| ArthritoMab™ arthritis-inducing antibody cocktail | MD Bioproducts | CIA-MAB-2C |
| Lipopolysaccharide (Escherichia Coli) | MD Bioproducts | MDLPS |
| Phosphate buffered saline | Waldeck GmBH & Co. KG | 3L-175 |
| Paraformaldehyde 4% | Merck KGaA | K51726405009 |
| Liquid nitrogen | Cryotherm GmBH & Co. KG | UN1977/ 78400571 |
| Ethanol 70% | Carl Roth GmBH & Co. KG | K928.4 |
| Experimental models: Organisms/strains | ||
| Mus musculus: Wild type (C57Bl/6J), adult (10–12 weeks), male | The Jackson Laboratory | 000664 |
| Other | ||
| Scale | KERN & Sohn GmbH | EMB Scale Ø 150 mm |
| Digital caliper | iGaging | OriginCal IP54 |
| BIO-GS3 Grip Strength Test | Bioseb | N/A |
| Sterican R Gr. 20, 27G | B. Braun SE | 4657705 |
| Omnifix R Solo, 1 mL | B. Braun SE | 9161309V |
| SAFE® compact crinklets natural | Safe diets | N/A |
Step-by-step method details
Monoclonal antibody cocktail and LPS injection
Timing: 3 min/mouse
In this paragraph, we describe the intraperitoneal (i.p.) application of the monoclonal antibody cocktail on day 0 and of LPS on day 3. Additionally, we highlight important 3R considerations. Some authors suggest intravenous (i.v.) application of the monoclonal antibody cocktail (Bas et al., 2012). We found the i.p. protocol to be favorable regarding handling for researchers and animals. In our view, the clinical course of RA is best mimicked by the i.p. method, as the disease onset is slightly slower and the peak less pronounced compared to the i.v. method, which is described to yield higher arthritis severity (Chondrex, 2022).
-
1.
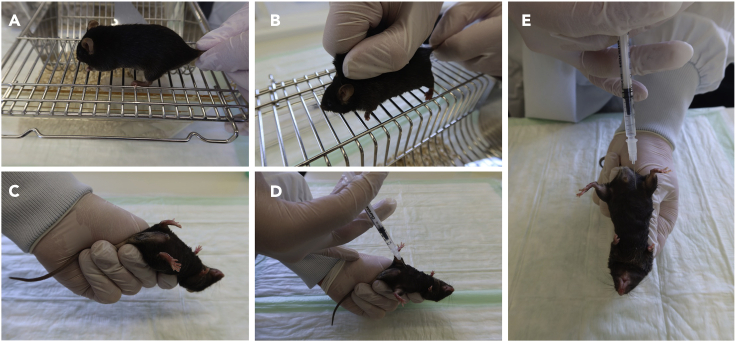
Place mouse on metal grid (e.g., part of your cage) and allow the animal to gain traction with all four paws, while holding its tail as proximal as possible with your dominant hand (Figure 2A).
-
2.Gently pull back the tail to allow the mouse to hold onto the grid.
-
a.Put your thumb and forefinger to the middle of the thoracic spine.
-
b.Carefully apply pressure and move your two fingers toward the neck (Figure 2B).
-
a.
-
3.
Grab loose skin at the back of the neck between thumb and forefinger. Hold tail with little and ring finger (Figure 2C).
Note: To ensure ideal handling grab as much skin as possible. By doing so, the abdominal skin will tighten and ease the injection process and reduce stress for mice.
-
4.
Hold the mouse with the abdomen facing upwards in a 45° angle and the head towards the table. (Figure 2D).
Note: Abdominal organs will move towards the diaphragm and an i.p. injection can now be performed safely.
-
5.Perform injection.
-
a.Aim for the lower right abdomen with the needle directed towards the thorax in a 30°–40° angle to minimize risk of puncturing intestines (Figure 2E).
-
b.Perforate skin and slowly aspirate. Check syringe for aspirated liquid.
-
i.Only apply antibody cocktail if no liquids are aspirated.
-
i.
-
c.After application, wait 5 s and slowly remove needle and syringe.
-
a.
Note: We recommend 27–30 G needles, as these diameters are less likely to perforate intestines and hurt the animal, compared to larger needles.
CRITICAL: Keeping the animal’s head down as well as injecting the solution slowly with an angle of 30°–40° will prevent antibody cocktail reflux from the injection site. Make sure not to move the needle inside the abdomen.
Note: Puncturing intestines may result in aspiration of green liquid, puncturing an abdominal vessel may result in aspiration of blood and perforating the bladder might lead to aspiration of urine. If any of this happens carefully withdraw and discard the syringe and refrain from a re-injection. Observe the animal for any signs of pain, illness, etc. during the following 48–72 h (UBC, 2014).
Note: If antibody cocktail reflux occurs after removal of the needle and syringe and exceeds a small drop, you may consider a premature termination of the experiment for the affected animal. As the CAIA model is dose-dependent, the animal may develop less severe arthritis and obtained results are not reliable anymore.
-
6.Put mouse in an extra cage to allow recovery until injections of other mice are completed.
-
a.Relocate all mice to their original cages.
-
a.
-
7.
For LPS injection on day 3, follow the same instructions (steps 1.–6.) using 100 μg (=200 μL) of LPS instead of the antibody cocktail.
Note: Avoid changing nesting material (this may include paper tissue, shredded paper, or if available SAFE® compact crinklets or alternative environmental enrichments) during the acute inflammatory arthritis phase (up to day 14). Mice with severe arthritis might not be able to build a new nest and rely on previously built nests. However, always consider hygiene aspects and potentially replace contaminated with pre-formed nests.
Figure 2.
Mouse handling for i.p. CAIA cocktail/LPS/PBS injection
(A–E) (A) Hold mouse at the proximal tail with your dominant hand, (B) use your non-dominant hand with your thumb and forefinger to firmly grab the animal’s skin, (C) turn the mouse to a supine position and (D and E) inject CAIA cocktail/LPS/PBS i.p. into the lower right abdominal quadrant.
Daily assessments
Timing: day 0 until euthanasia
In this paragraph we elaborate on daily arthritis assessments including the clinical semiquantitative arthritis score, ankle size measurements and grip strength. For details on execution of these methods, please refer to (Maleitzke et al., 2021, 2022).
-
8.
Check health status and body weight (Figure 3).
Note: Following CAIA induction, mice are expected to lose weight until they reach the clinical peak of arthritis around day 8–12. Following LPS injection, mice can lose up to 20–30% of their body weight. Within 24–48 h mice should however start re-gaining weight. Mice with severe loss of body weight without rapid recovery (24–48 h) or signs of reduced overall well-being should immediately be checked by a veterinarian. If deemed necessary, the experiment must be terminated prematurely at this point.
-
9.Clinical arthritis assessment employing the semiquantitative arthritis score.
-
a.Grab mouse as described in steps 1.–3. (Figures 2A–2C).
-
b.Give each paw a score between 0–3 based on swelling and erythema (Lee et al., 2006):
-
i.0: No swelling; 1: Mild to moderate swelling and erythematic ankle and/or 1 swollen digit; 2: Moderate swelling and erythematic ankle or swelling in 2 or more digits; 3: Marked swelling along all aspects of the paw or all 5 digits swollen.
-
i.
-
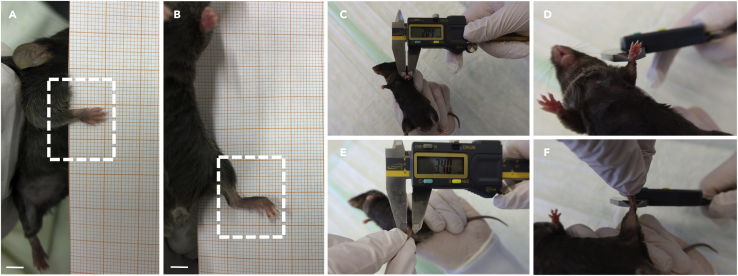
c.Place the paw you are scoring on scale paper and apply gentle pressure.
-
d.Take images for documentation (Figures 4A and 4B).
-
a.
Note: Placing the paw on scale paper while applying gentle pressure will cause the mouse to spread its digits, which allows an easy evaluation of each digit and the paw as a whole.
- 10.
Note: Pay attention not to injure the animal’s skin with the caliper. In our experience, measuring ankle and wrist size does often not create reproducible results. Due to the high inter-observer variance, it should only be conducted in addition to clinical arthritis scoring and repeated, if possible, to acquire a representative mean value.
-
11.To assess pain and functionality in forelimbs we use a grip strength test with a metal grasping grid (Bioseb, 2022).
-
a.Grab the mouse by its proximal tail.
-
b.Place the animal on the metal grid only allowing its forelimbs to grasp the grid (Figure 5A).
-
c.Gently pull the animal backwards horizontally until it lets go of the grid. Document the value indicated on the screen (Figure 5B).
-
d.Repeat measurements five times and calculate mean or maximum values.
-
e.Keep mice in cages between measurements.
-
a.
Note: Placing all four limbs on the grid and performing grip strength tests is possible too. The grip strength test measures pain-induced functional impediments, therefore its reliability is reduced when using analgesics (Montilla-Garcia et al., 2017). This should be considered in your experimental design.
Figure 3.
Daily body weight assessment
Daily assessment of body weight is conducted with a regular laboratory scale and glass container.
Figure 4.
Daily assessments of semi-quantitative arthritis score and ankle size measurements
(A–F) (A) Place front and (B) back paws on scale paper to document arthritis severity. Measure (C and D) front and (E and F) back paws with a digital caliper at the maximum diameter of wrist and ankle. Scale bars 500 μm.
Figure 5.
Daily assessment of grip strength
(A) Grip strength assessment is conducted using a commercially available electronic device with a metal grasping grid for the mouse to hold on to with its front paws.
(B) Gently pull the animal backwards horizontally until it lets go.
Joint extraction
Timing: day 10/48, 20 min/mouse
In this section we introduce an overview of tissue harvesting, relevant to most murine arthritis experiments. Tissues are harvested at two different endpoints (day 10 and 48) which serves the purpose of analyzing (i) the acute inflammatory and (ii) the chronic resolution phase of arthritis.
-
12.
Euthanize mice depending on guidelines provided by your local animal welfare authorities.
-
13.Carefully dissect all extremities.
-
a.Strip left side limbs of all muscle and soft tissue.
-
b.Separate wrist, ankle, and knee joints. Leave joint capsules intact.
-
i.Snap freeze joint samples in liquid nitrogen for further molecular analysis (e.g., mRNA or protein expression) (Figure 6A).
-
i.
-
c.Strip right side limbs from skin.
-
d.Separate and fixate right side wrist, ankle, and knee joints in paraformaldehyde (PFA) 4% for 12–24 h at 4°C. (Figure 6A).
-
i.Wash out and replace PFA with PBS before micro-computed tomography (μCT) analysis, which is usually followed by embedding joint samples for histological assessment.
-
i.
-
a.
Note: When removing skin and muscle, pay attention to not strip joints of periarticular tissue, as this may destroy the joint capsule and impair further results. Arthritis manifests peri- and intraarticularly, therefore it is advised to preserve these structures for all analyses.
Note: To distinguish local from systemic arthritis effects on bone and cartilage, we suggest analyzing subchondral bone areas regarding local changes (e.g., talus) and trabecular bone areas (e.g., proximal tibia) for systemic bone alterations. We recommend assessing wrist, ankle, and knee joints as they have all been previously decribed extensively in numerous publications (Bendele et al., 1999; Khachigian, 2006; Lee et al., 2006; Spies et al., 2014; Tu et al., 2016).
Note: Besides harvesting joints and bones, it is recommended to extract blood (serum or plasma) for biomarker assessments through intracardiac puncture following euthanasia. Further tissues to be harvested may include muscle and visceral organs according to specific study objectives (Figure 6A).
Pause point: Harvested tissues can be stored in PBS at 4°C for several days to weeks after PFA fixation for at least 12 h. Snap frozen and embedded tissues can be stored at −80°C for extended periods.
Note: Avoid freeze–thaw cycles.
Figure 6.
Schematic diagram for tissue harvesting and overview of clinical, radiological, and histological outcomes
(A) Proposed distribution of joint and serum samples for radiological, histological, and molecular analyses.
(B) Exemplified semi-quantitative arthritis score and grip strength over the course of 48 days when comparing wild type animals to a potential arthritis altering intervention.
(C–E) Representative images of (C) arthritic ankle swelling on day 6 (white arrows), (D) loss of trabecular bone integrity of proximal tibiae in μCT on day 48 (white arrow), and (E) severe intra- and periarticular inflammation of knee joint sections (hematoxylin and eosin-stained) on day 10 (white boxes) following CAIA induction vs. control/sham. (B) created with BioRender.com. Scale bars 500 μm.
Expected outcomes
The clinical semiquantitative arthritis score, ankle size measurements and grip strength assessments usually serve the purpose of comparing two experimental groups exposed to CAIA (e.g., knockout vs. WT; drug vs. vehicle). One group commonly shows less severe signs of arthritis, resulting in lower clinical arthritis scores, a smaller ankle size and preserved grip strength (Figures 6B and 6C).
Bone changes may be assessed by local porosity parameters and systemic trabecular parameters, using μCT technology. Different experimental groups commonly show different bone constitutions, which are either protected from arthritic bone changes or show a full arthritic phenotype, following exposure to CAIA (Figure 6D).
To confirm clinical results, histology and histomorphometry help to assess the intraarticular environment altered by arthritis. Hematoxylin and eosin and toludine blue stainings aid to assess inflammation and cartilage degradation (Figure 6E). Usually different experimental groups then show different (higher/lower) histology scores. Tartrate-resistant acid phosphatase (TRAP) staining and histomorphometry help to evaluate bone erosions, osteoclast patterns and subchondral bone remodeling. Immunohistochemistry allows to confirm expression of target proteins intraarticularly.
RNA analyses by real-time polymerase chain reaction (RT-PCR) can be used to assess gene expressions in joints and organ samples obtained from experimental animals exposed to CAIA compared to control animals. In accordance with previous outcome parameters, one group may show elevated or reduced expressions of inflammatory, immune, bone and/or cartilage turnover markers.
Serum/plasma analysis by Enzyme-linked Immunosorbent Assay (ELISA) provides information on systemic protein/biomarker expressions which may be elevated or reduced in experimental groups compared to controls following CAIA induction.
Limitations
In the present protocol the development of arthritis is induced by i.p. administration of autoantibodies directed against type II collagen, followed by an i.p. boost of LPS three days later.
Compared to the traditionally used collagen-induced arthritis (CIA) model, the CAIA model facilitates a rapid onset of disease within 48 h, reaching its peak within eight days instead of multiple weeks (Terato et al., 1992). The disease shows an arthritis incidence of nearly 100% even in non-CIA-susceptible mouse strains including genetically modified mice. However, costs are higher and the rapid development of arthritis does not mimic the chronic progression typically observed in human disease (Khachigian, 2006).
Besides being simpler in execution and allowing a slower disease onset, the i.p. injection of the monoclonal antibody cocktail might result in less severe arthritis and a shorter period of active inflammation compared to the i.v. administration (Chondrex, 2022). If injected without care, antibody cocktail reflux from the injection site may leave animals with an insufficient amount of antibodies, which should lead to the exclusion of the animal from the experiment, as the CAIA model is dose-dependent.
Since LPS is required for the CAIA model, mice must be held in a specific pathogen-free environment to avoid bacterial contamination. Further, analgesia has a direct effect on the data obtained from the grip strength test (Montilla-Garcia et al., 2017).
Finally, as exposure to estrogen (in female mice) seems to modulate and partially attenuate CAIA, the model works best in adult male mice (Nandakumar et al., 2003).
Troubleshooting
Problem 1
Arthritis does not develop.
Potential solution
Conducting titration experiments may be necessary, as mouse strains react differently to different antibody cocktail doses. LPS dose should not be increased. See preparation of monoclonal antibody cocktail injection (day 0), Note.
Store antibody cocktail at −20°C and protect from direct light. Avoid redundant freeze–thaw cycles to maintain antibody quality. Therefore, consider storing CAIA cocktail in small aliquots. See preparation of monoclonal antibody cocktail injection (day 0), step 5.
Problem 2
Reflux of antibody cocktail from the injection site after withdrawing the syringe from the abdominal cavity.
Potential solution
Holding the head of the mouse downwards, injecting slowly and steadily, and waiting 5 s before withdrawing the needle will ensure injected antibody cocktail has enough time to disperse i.p. See step-by-step method details, steps 4 and 5.
Problem 3
Not being able to hold the mouse properly and thus increasing the risk of puncturing abdominal organs and injuring mice during ankle size measuring.
Potential solution
Mice should be accustomed to the investigator prior to first injection. Therefore, ensure an accommodation time of 14 days. Slowly moving fingers from the back towards the neck with thumb and index finger will give you a better control over the mouse and allow an optimal grip of the skin on the back of the neck. See step-by-step method details, steps 2 and 3.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be provided by the lead contact, Johannes Keller (j.keller@uke.de).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by grants of the Stiftung Oskar-Helene-Heim (OHH_44) to T.M., the Else Kröner-Fresenius-Stiftung (EKFS 2017_A22) to J.K., and the Deutsche Forschungsgemeinschaft (DFG KE 2179/2-1, TS 303/2-1) to S.T. and J.K. T.M. is a participant in the BIH Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH).
The authors are grateful for the assistance provided by Juliane Unger and her team of animal caretakers at the Forschungseinrichtung für Experimentelle Medizin (FEM, Charité – Universitätsmedizin Berlin, Germany).
Author contributions
T.M. and J.W. wrote the current protocol. J.W., A.H., T.D., and S.Z. provided and prepared images. T.M., S.T., and J.K. provided supervision. All authors critically revised the protocol and agreed to its submission.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate/analyze datasets/code.
References
- Bas D.B., Su J., Sandor K., Agalave N.M., Lundberg J., Codeluppi S., Baharpoor A., Nandakumar K.S., Holmdahl R., Svensson C.I. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum. 2012;64:3886–3896. doi: 10.1002/art.37686. [DOI] [PubMed] [Google Scholar]
- Bendele A., McAbee T., Sennello G., Frazier J., Chlipala E., McCabe D. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: comparison of efficacy in animal models with human clinical data. Arthritis Rheum. 1999;42:498–506. doi: 10.1002/1529-0131(199904)42:3<498::AID-ANR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Benson R.A., McInnes I.B., Garside P., Brewer J.M. Model answers: rational application of murine models in arthritis research. Eur. J. Immunol. 2018;48:32–38. doi: 10.1002/eji.201746938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioseb BIO-GS3 grip strength test. 2022. https://www.bioseb.com/en/activity-motor-control-coordination/48-grip-strength-test.html
- Caplazi P., Baca M., Barck K., Carano R.A.D., DeVoss J., Lee W.P., Bolon B., Diehl L. Mouse models of rheumatoid arthritis. Vet. Pathol. 2015;52:819–826. doi: 10.1177/0300985815588612. [DOI] [PubMed] [Google Scholar]
- Chondrex Collagen antibody induced arthritis (CAIA) in mice. 2022. https://www.chondrex.com/documents/Mouse-CAIA.pdf
- Khachigian L.M. Collagen antibody-induced arthritis. Nat. Protoc. 2006;1:2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- Lee H., Zahra D., Vogelzang A., Newton R., Thatcher J., Quan A., So T., Zwirner J., Koentgen F., Padkjaer S.B., Mackay C.R. Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat. Biotechnol. 2006;24:1279–1284. doi: 10.1038/nbt1248. [DOI] [PubMed] [Google Scholar]
- Maleitzke T., Hildebrandt A., Dietrich T., Appelt J., Jahn D., Otto E., Zocholl D., Baranowsky A., Duda G.N., Tsitsilonis S., Keller J. The calcitonin receptor protects against bone loss and excessive inflammation in collagen antibody-induced arthritis. iScience. 2022;25:103689. doi: 10.1016/j.isci.2021.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleitzke T., Hildebrandt A., Weber J., Dietrich T., Appelt J., Jahn D., Zocholl D., Baranowsky A., Duda G.N., Tsitsilonis S., Keller J. Proinflammatory and bone protective role of calcitonin gene-related peptide alpha in collagen antibody-induced arthritis. Rheumatology. 2021;60:1996–2009. doi: 10.1093/rheumatology/keaa711. [DOI] [PubMed] [Google Scholar]
- Matzelle M.M., Gallant M.A., Condon K.W., Walsh N.C., Manning C.A., Stein G.S., Lian J.B., Burr D.B., Gravallese E.M. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2012;64:1540–1550. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDBioproducts ArthritoMab™ arthritis inducing antibody Cocktail (for use in anti-collagen antibody induced arthritis (CAIA) 2022. https://cdn.shopify.com/s/files/1/0554/5485/9453/files/CIA-MAB-50_Insert_R1221_92db8f2d-ce37-4022-bbfc-1b463cdd43c9.pdf?v=1649066407
- Montilla-García Á., Tejada M.Á., Perazzoli G., Entrena J.M., Portillo-Salido E., Fernández-Segura E., Cañizares F.J., Cobos E.J. Grip strength in mice with joint inflammation: a rheumatology function test sensitive to pain and analgesia. Neuropharmacology. 2017;125:231–242. doi: 10.1016/j.neuropharm.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Nandakumar K.S., Bäcklund J., Vestberg M., Holmdahl R. Collagen type II (CII)-specific antibodies induce arthritis in the absence of T or B cells but the arthritis progression is enhanced by CII-reactive T cells. Arthritis Res. Ther. 2004;6:R544–R550. doi: 10.1186/ar1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar K.S., Holmdahl R. Antibody-induced arthritis: disease mechanisms and genes involved at the effector phase of arthritis. Arthritis Res. Ther. 2006;8:223. doi: 10.1186/ar2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar K.S., Svensson L., Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am. J. Pathol. 2003;163:1827–1837. doi: 10.1016/s0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun P., Claudio E., Ren N., Wang H., Siebenlist U. The adaptor protein CIKS/ACT1 is necessary for collagen-induced arthritis, and it contributes to the production of collagen-specific antibody. Arthritis Rheum. 2010;62:3334–3344. doi: 10.1002/art.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pérez D., Benito J., Largo C., Polo G., Canfrán S., Gómez de Segura I.A. Metamizole (dipyrone) effects on sevoflurane requirements and postoperative hyperalgesia in rats. Lab. Anim. 2017;51:365–375. doi: 10.1177/0023677216671553. [DOI] [PubMed] [Google Scholar]
- Schinnerling K., Rosas C., Soto L., Thomas R., Aguillón J.C. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front. Immunol. 2019;10:203. doi: 10.3389/fimmu.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies C.M., Wiebe E., Tu J., Li A., Gaber T., Huscher D., Seibel M.J., Zhou H., Buttgereit F. Acute murine antigen-induced arthritis is not affected by disruption of osteoblastic glucocorticoid signalling. BMC Musculoskelet. Disord. 2014;15:31. doi: 10.1186/1471-2474-15-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terato K., Hasty K.A., Reife R.A., Cremer M.A., Kang A.H., Stuart J.M. Induction of arthritis with monoclonal antibodies to collagen. J. Immunol. 1992;148:2103–2108. https://www.ncbi.nlm.nih.gov/pubmed/1545120 [PubMed] [Google Scholar]
- Tu J., Zhang Y., Kim S., Wiebe E., Spies C.M., Buttgereit F., Cooper M.S., Seibel M.J., Zhou H. Transgenic disruption of glucocorticoid signaling in osteoblasts attenuates joint inflammation in collagen antibody-induced arthritis. Am. J. Pathol. 2016;186:1293–1301. doi: 10.1016/j.ajpath.2015.12.025. [DOI] [PubMed] [Google Scholar]
- UBC Intraperitoneal (IP) injection in rats and mice SOP. 2014. https://animalcare.ubc.ca/sites/default/files/documents/TECH%2010%20IP%20Injections%20in%20the%20Mouse%20and%20Rat.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.