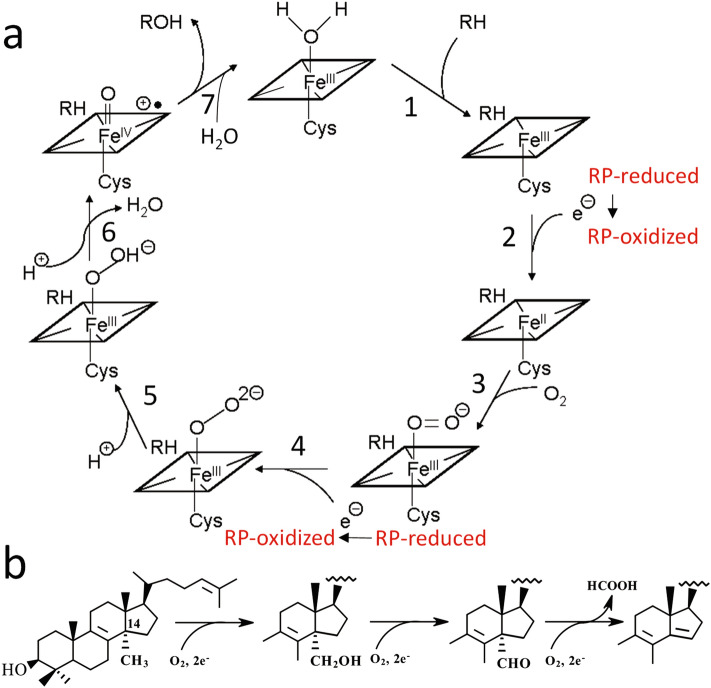
Figure 1.
(a) Cytochrome P450 catalytic cycle. The cycle begins with the binding of a substrate, which increases the redox potential of the ferric heme iron (1). The iron (FeIII) accepts the first electron from the redox partner protein [RP], and the redox complex dissociates (2). The ferrous iron (FeII) binds molecular oxygen (3). The dioxygen complex accepts the second electron from the RP producing the ferric-peroxo anion and the redox complex dissociates (4). The ferric-peroxo anion is then protonated to form the ferric-hydroperoxo state (5). The second protonation of the distal oxygen atom causes the O–O bond scission, release of a water molecule, and generation of a highly reactive electrophilic ferryl (FeIV)-oxo cation radical known as Compound I (6), leading to insertion of the second oxygen atom into the substrate (7). (b) CYP51 reaction includes three P450 cycles and thus requires six sequential electron transfer events.