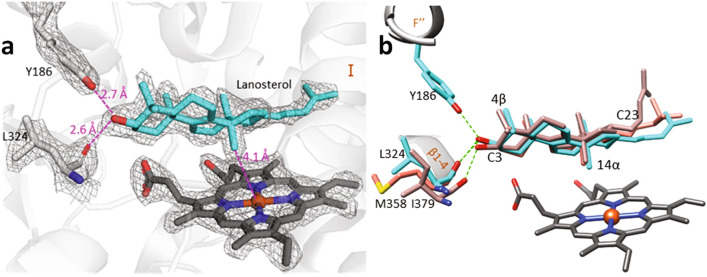
Figure 3.
Binding mode of the CYP51 substrate is conserved across phylogeny. (a) The 2Fo-Fc electron density map (at 2σ) for lanosterol, the heme, and the H-bond forming residues, Y186 and L324, in the structure of M. capsulatus CYP51. (b) Sterol substrates bound in the active center of bacterial (M. capsulatus—cyan, and eukaryotic CYP51s (human—rosy-brown, and T. cruzi—salmon). The residues preceding the β1-4 strand (L324, I379, and M358) whose main chain carbonyl oxygen forms the H-bond with the sterol hydroxyl are colored correspondingly. The distances and the H-bonds are depicted as magenta and green dashes, respectively.