Abstract
Background
The systemic immune-inflammation index (SII) is a novel marker of inflammation, and hepatic steatosis and fibrosis are associated with inflammation. This study aimed to investigate the possible relationship between SII and hepatic steatosis and fibrosis.
Methods
The datasets from the National Health and Nutrition Examination Survey (NHANES) 2017–2020 were used in a cross-sectional investigation. Multivariate linear regression models were used to examine the linear connection between SII and controlled attenuation parameter (CAP) and liver stiffness measurement (LSM). Fitted smoothing curves and threshold effect analysis were used to describe the nonlinear relationship.
Results
This population-based study included a total of 6,792 adults aged 18–80 years. In a multivariate linear regression analysis, a significant positive association between SII and CAP was shown [0.006 (0.001, 0.010)]. This positive association in a subgroup analysis was maintained in men [0.011 (0.004, 0.018)] but not in women. Furthermore, the association between SII and CAP was nonlinear; using a two-segment linear regression model, we found an inverted U-shaped relationship between SII and CAP with an inflection point of 687.059 (1,000 cells/µl). The results of the multiple regression analysis showed that the relationship between SII and LSM was not significant (P = 0.263).
Conclusions
Our findings imply that increased SII levels are linked to hepatic steatosis, but SII is not linked to liver fibrosis. To confirm our findings, more large-scale prospective investigations are needed.
Keywords: systemic immune-inflammatory index, NAFLD, NHANES, hepatic steatosis, liver fibrosis
Background
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide and one of the primary causes of severe liver disease (1–3). NAFLD is defined as excessive fat infiltration into the liver in the absence of substantial alcohol intake or secondary causes (4), which includes a variety of histological alterations in the liver, ranging from simple steatosis through leukocyte infiltration and hepatocyte ballooning to severe liver fibrosis and cirrhosis (5, 6). Transient elastography is widely used in the screening of NAFLD due to its good accuracy and noninvasive feature (7, 8); controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) were used to assess hepatic steatosis and fibrosis, respectively (9, 10).
The systemic immune-inflammation index (SII) is an integrated and novel inflammatory biomarker as reported in the study by Hu et al. (11) in 2014, which could reflect the local immune response and systemic inflammation in the whole human body (12–15). SII has been used in past studies to predict and evaluate the prognosis of various solid tumors, such as gastric cancer (16, 17), non-small cell lung cancer (18, 19), pancreatic cancer (20), and esophageal cancer (21, 22). In addition, SII also has a high value for the prognosis of cardiovascular disease (23–27). Inflammation is a hallmark of NAFLD progression, and the recruitment of circulating inflammatory cells and the upregulation of inflammatory mediators play an important role in hepatic steatosis and fibrosis (28–31). Fontes-Cal et al. (32) reported that plasma cytokines and clinical parameters of inflammation could serve as a new strategy for monitoring NAFLD progression. However, the relationship between SII and hepatic steatosis and fibrosis remains unclear.
As a result, we examined the relationship between SII and CAP and LSM in adults in this study, utilizing a large sample of people aged 18 to 80 years from the National Health and Nutrition Examination Survey (NHANES).
Methods
Study population
The NHANES is a representative survey of the US national population that uses a complicated, multistage, and probabilistic sampling methodology to provide a wealth of information about the general US population’s nutrition and health (33). The 2017–2020 continuous cycle of the US NHANES dataset was used for this investigation. We excluded 3,409 participants with missing SII data, 2,941 with missing CAP or LSM data, 90 hepatitis B antigen-positive and 132 hepatitis C antibody-positive or hepatitis C RNA-positive samples, 959 participants with significant alcohol consumption (ever have 4, 5, or more drinks every day), and 1,237 participants younger than 18 years from the 15,560 eligible individuals. The study eventually included 6,792 participants. Figure 1 illustrates the sample selection flowchart.
Figure 1.
Flowchart of participant selection. NHANES, National Health and Nutrition Examination Survey; SII, systemic immune-inflammation index; CAP, controlled attenuation parameter; LSM, liver stiffness measurement.
Study variables
The dependent variable in this study is the systemic immune-inflammation index, with CAP and LSM as the intended independent variables. In our analysis, SII was designed as an exposure variable. Lymphocyte, neutrophil, and platelet counts were measured by complete blood count using automated hematology analyzing devices (Coulter®DxH 800 analyzer) and presented as ×103 cells/ml. SII as an exposure variable was derived from platelet count × neutrophil count/lymphocyte count (11, 13, 34). CAP and LSM were designed as outcome variables to measure hepatic steatosis and liver fibrosis. The NHANES staff evaluated participants for Vibration controlled transient elastography (VCTE) using the FibroScan®-equipped model 502 V2 Touch. According to a recent landmark study, CAP values, also known as CAP, ≥274 dB/m was considered indicative of NAFLD status because of 90% sensitivity in detecting all degrees of hepatic steatosis (9). Based on two past studies, CAP ≥302 dB/m was defined in this study as having severe steatosis at the base of NAFLD (4, 35). Fibrosis grade was determined by liver stiffness with cutoff values of 8.2, 9.7, and 13.6 kPa for fibrosis grades ≥F2, ≥F3, and F4, respectively, and was optimized using the Jorden index (36, 37). Covariates included age, gender, race, Body Mass Index (BMI), education level, family income-to-poverty ratio, activity status, alanine transaminase (ALT), weight, alkaline phosphatase (ALP), waist circumference, aspartate aminotransferase (AST), total calcium, total cholesterol, direct High-Density Lipoprotein Cholesterol (HDL-C), Low-Density Lipoprotein Cholesterol (LDL-C), triglyceride, serum phosphorus, and smoking status.
Statistical analysis
The statistical study was carried out using the statistical computing and graphics software R (version 4.1.3) and EmpowerStats (version:2.0). Baseline tables for the study population were statistically described by CAP and LSM subgroups; continuous variables are described using mean values plus or minus standard deviation (SD) and weighted linear regression models. The beta values and 95% confidence intervals were calculated using multivariate linear regression analysis between the SII and CAP and LSM. The multivariate test was built using three models: model 1: no variables adjusted; model 2: gender, age, and race adjusted; model 3: adjusted for all covariates. By adjusting the variables, smoothed curve fits were done simultaneously. A threshold effects analysis model was used to examine the relationship and inflection point between SII and CAP. Finally, the same statistical study methods described above were conducted for the gender subgroups. It was determined that P < 0.05 was statistically significant. We used a weighting approach to reduce the significant volatility of our dataset.
Results
Baseline characteristics
In this study, 6,792 adults were included based on the inclusion and exclusion criteria, and the average age of the participants was 48.58 ± 18.50 years. Among these participants, 45.39% were men, 54.61% were women, 33.82% were non-Hispanic white, 25.28% were non-Hispanic black, and 12.38% were Mexican American, and 28.52% were from other races. The mean (SD) concentrations of CAP, LSM, and SII were 262.49 (62.84) dB/m, 5.84 (4.81) kPa, and 515.48 (341.66) (1,000 cells/µl), respectively.
Table 1 lists all clinical characteristics of the participants with CAP as a column-stratified variable. In comparison to the non-NAFLD group, the severe steatosis group is more likely to be men and older, with a higher proportion of non-Hispanic blacks and Mexican Americans; with higher smoking status; and higher levels of BMI, waist circumference, AST, ALT, ALP, total cholesterol, LDL cholesterol, triglyceride, LSM, and SII but lower levels of direct HDL cholesterol and serum phosphorus.
Table 1.
Weighted characteristics of the study population based on controlled attenuated parameter (CAP).
| Non-NAFLD (CAP < 274, n = 3,901) | NAFLD (274 ≤ CAP < 302, n = 1,031) | Severe steatosis (CAP ≥ 302, n = 1,860) | P value | |
|---|---|---|---|---|
| Age (years) | 46.135 ± 19.305 | 51.607 ± 17.552 | 52.013 ± 16.389 | <0.001 |
| Gender (%) | <0.001 | |||
| Men | 41.989 | 43.938 | 53.333 | |
| Women | 58.011 | 56.062 | 46.667 | |
| Race/Ethnicity (%) | <0.001 | |||
| Non-Hispanic White | 33.017 | 32.590 | 36.183 | |
| Non- Hispanic Black | 28.198 | 24.151 | 19.785 | |
| Mexican American | 9.510 | 14.646 | 17.151 | |
| Other Race | 29.275 | 28.613 | 26.882 | |
| Education lever (%) | 0.089 | |||
| Less than high school | 16.597 | 18.924 | 18.932 | |
| High school | 22.555 | 22.809 | 23.720 | |
| More than high school | 60.848 | 58.267 | 57.348 | |
| Moderate activities (%) | <0.001 | |||
| Yes | 46.091 | 40.543 | 37.312 | |
| No | 53.909 | 59.457 | 62.688 | |
| Smoked at least 100 cigarettes | <0.001 | |||
| Yes | 32.479 | 31.620 | 40.430 | |
| No | 67.521 | 68.380 | 59.570 | |
| Income to poverty ratio | 2.646 ± 1.663 | 2.716 ± 1.601 | 2.649 ± 1.608 | 0.520 |
| BMI (kg/m2) | 26.902 ± 5.946 | 31.480 ± 6.801 | 34.873 ± 7.725 | <0.001 |
| Waist circumference (cm) | 92.211 ± 14.260 | 104.038 ± 13.584 | 112.974 ± 15.613 | <0.001 |
| Laboratory features | ||||
| Total calcium (mmol/L) | 2.320 ± 0.092 | 2.320 ± 0.098 | 2.318 ± 0.097 | 0.673 |
| Total cholesterol (mmol/L) | 4.714 ± 1.024 | 4.890 ± 1.045 | 4.848 ± 1.054 | <0.001 |
| Triglyceride(mmol/L) | 0.979 ± 0.727 | 1.379 ± 1.552 | 1.629 ± 1.193 | <0.001 |
| LDL- cholesterol(mmol/L) | 2.750 ± 0.891 | 2.903 ± 0.932 | 2.868 ± 0.927 | <0.001 |
| HDL- cholesterol(mmol/L) | 1.474 ± 0.400 | 1.336 ± 0.392 | 1.211 ± 0.342 | <0.001 |
| ALT (IU/L) | 18.311 ± 13.825 | 22.347 ± 15.461 | 27.978 ± 20.136 | <0.001 |
| AST (IU/L) | 20.302 ± 10.862 | 21.414 ± 12.840 | 23.287 ± 14.532 | <0.001 |
| ALP(IU/L) | 74.800 ± 24.197 | 78.789 ± 22.622 | 81.974 ± 25.317 | <0.001 |
| Serum phosphorus (mmol/L) | 1.162 ± 0.166 | 1.149 ± 0.164 | 1.137 ± 0.169 | <0.001 |
| LSM (kPa) | 5.122 ± 3.873 | 5.895 ± 4.414 | 7.328 ± 6.229 | <0.001 |
| SII (1,000 cells/µl) | 509.876 ± 364.567 | 504.868 ±289.827 | 533.097 ± 317.184 | 0.030 |
Mean ± SD for continuous variables: P value was calculated by weighted linear regression model.
% for categorical variables: P value was calculated by weighted chi-square test.
BMI, body mass index; LDL- cholesterol, low-Density Lipoprotein Cholesterol; HDL- cholesterol, high-Density Lipoprotein Cholesterol; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; LSM, liver stiffness measure ; CAP, controlled attenuation parameter; SII, systemic immune-inflammation index.
Table 2 lists all clinical features of the individuals with LSM as a column-stratified variable. In comparison to the normal group, the cirrhosis group is more likely to be men and older, with a higher proportion of non-Hispanic blacks and Mexican Americans; with higher smoking status; and higher levels of BMI, waist circumference, AST, ALT, ALP, LDL cholesterol, triglyceride, CAP, and SII but lower levels of HDL cholesterol, total cholesterol, and serum phosphorus.
Table 2.
Weighted characteristics of the study population based on median liver stiffness measurement (LSM).
| Normal group(LSM<8.2, n = 6,098) | Significant fibrosis(8.0≤LSM<9.7, n = 283) | Advanced fibrosis(9.7≤LSM<13.6, n = 223) | Cirrhosis(LSM≥13.6, n = 188) | P value | |
|---|---|---|---|---|---|
| Age (years) | 47.859 ± 18.578 | 53.484 ± 16.578 | 56.309 ± 16.466 | 55.250 ± 16.525 | <0.001 |
| Gender (%) | <0.001 | ||||
| Men | 44.654 | 49.823 | 49.776 | 57.447 | |
| Women | 55.346 | 50.177 | 50.224 | 42.553 | |
| Race/Ethnicity (%) | 0.018 | ||||
| Non-Hispanic White | 33.585 | 31.449 | 38.565 | 39.362 | |
| Non- Hispanic Black | 25.057 | 32.509 | 24.215 | 22.872 | |
| Mexican American | 12.283 | 12.014 | 12.108 | 16.489 | |
| Other Race | 29.075 | 24.028 | 25.112 | 21.277 | |
| Education lever (%) | 0.898 | ||||
| Less than high school | 17.405 | 19.343 | 18.894 | 20.330 | |
| High school | 22.668 | 24.818 | 26.267 | 24.176 | |
| More than high school | 59.927 | 55.839 | 54.839 | 55.495 | |
| Moderate activities (%) | 0.003 | ||||
| Yes | 43.736 | 36.396 | 33.632 | 34.574 | |
| No | 56.264 | 63.604 | 66.368 | 65.426 | |
| Smoked at least 100 cigarettes | 0.081 | ||||
| Yes | 33.864 | 36.749 | 44.843 | 40.426 | |
| No | 66.136 | 63.251 | 55.157 | 59.574 | |
| Income to poverty ratio | 2.669 ± 1.649 | 2.642 ± 1.580 | 2.504 ± 1.511 | 2.482 ± 1.514 | 0.270 |
| BMI (kg/m2) | 29.022 ± 6.691 | 33.734 ± 9.207 | 38.043 ± 9.651 | 38.538 ± 11.549 | <0.001 |
| Waist circumference (cm) | 97.955 ± 15.952 | 109.169 ± 19.514 | 119.096 ± 16.628 | 120.849 ± 20.775 | <0.001 |
| Laboratory features | |||||
| Total calcium (mmol/L) | 2.320 ± 0.093 | 2.321 ± 0.106 | 2.313 ± 0.109 | 2.307 ± 0.100 | 0.219 |
| Total cholesterol (mmol/L) | 4.792 ± 1.027 | 4.666 ± 1.104 | 4.720 ± 1.156 | 4.537 ± 1.115 | 0.002 |
| Triglyceride(mmol/L) | 1.194 ± 1.027 | 1.389 ± 1.353 | 1.597 ± 1.708 | 1.353 ± 0.902 | <0.001 |
| LDL- cholesterol(mmol/L) | 2.819 ± 0.897 | 2.733 ± 0.999 | 2.752 ± 1.067 | 2.547 ± 0.940 | 0.025 |
| HDL- cholesterol(mmol/L) | 1.395 ± 0.397 | 1.276 ± 0.351 | 1.247 ± 0.428 | 1.238 ± 0.449 | <0.001 |
| ALT (IU/L) | 20.657 ± 14.773 | 26.575 ± 19.780 | 30.677 ± 27.380 | 33.253 ± 32.692 | <0.001 |
| AST (IU/L) | 20.553 ± 9.249 | 24.085 ± 16.192 | 26.926 ± 19.327 | 34.086 ± 40.412 | <0.001 |
| ALP(IU/L) | 76.325 ± 23.230 | 82.905 ± 26.756 | 85.604 ± 30.118 | 93.269 ± 39.714 | <0.001 |
| Serum phosphorus (mmol/L) | 1.155 ± 0.166 | 1.148 ± 0.174 | 1.131 ± 0.174 | 1.131 ± 0.171 | 0.045 |
| CAP (dB/m) | 257.063 ± 60.343 | 297.473 ± 62.943 | 316.704 ± 61.176 | 321.665 ± 66.575 | <0.001 |
| SII (1,000 cells/µl) | 513.208 ± 335.813 | 537.926 ± 463.726 | 519.919 ± 322.459 | 549.945 ± 335.262 | 0.328 |
Mean ± SD for continuous variables: P value was calculated by weighted linear regression model.
% for categorical variables: P value was calculated by weighted chi-square test.
BMI, body mass index; LDL- cholesterol, low-Density Lipoprotein Cholesterol; HDL- cholesterol, high-Density Lipoprotein Cholesterol; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; LSM, liver stiffness measure ; CAP, controlled attenuation parameter; SII, systemic immune-inflammation index.
Association between systemic immune-inflammation index (SII) and controlled attenuation parameter (CAP)
Table 3 showed the results of the multivariate regression analysis. In the unadjusted model [0.006 (0.001, 0.010)], SII was highly associated with CAP. However, after adjusting for gender, age, and race variables, this significant positive correlation became insignificant in model 2 [0.002 (-0.002, 0.007)]. After adjusting for all covariates, the relationship between SII and CAP became negative in model 3 [-0.002 (-0.009, 0.004)].
Table 3.
The association between SII and CAP.
| Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | |
|---|---|---|---|
| CAP (dB/m) | 0.006 (0.001, 0.010) | 0.002 (-0.002, 0.007) | -0.002 (-0.009, 0.004) |
| 0.011 | 0.285 | 0.443 | |
| Stratified by CAP | |||
| Non-NAFLD | Reference | Reference | Reference |
| NAFLD | 0.004 (0.000, 0.009) | 0.005 (0.000, 0.009) | -0.003 (-0.010, 0.003) |
| 0.041 | 0.030 | 0.335 | |
| Severe steatosis | 0.003 (0.001, 0.008) | 0.005 (0.001, 0.010) | -0.002 (-0.008, 0.002) |
| 0.012 | 0.026 | 0.518 | |
| Stratified by gender | |||
| Men | 0.011 (0.004, 0.018) | 0.004 (-0.004, 0.011) | -0.003 (-0.013, 0.007) |
| 0.003 | 0.325 | 0.596 | |
| Women | 0.003 (-0.003, 0.008) | 0.002 (-0.003, 0.007) | -0.003 (-0.011, 0.005) |
| 0.335 | 0.470 | 0.491 | |
Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, educational level, BMI, family income-to-poverty ratio, moderate activities, smoking status, ALP, ALT, AST, total calcium, total cholesterol, triglyceride, LDL, HDL-C, waist circumference, and serum phosphorus were adjusted.
In the subgroup analysis stratified by gender and race, the model is not adjusted for sex and race, respectively.
NAFLD, non-alcoholic fatty liver disease; CAP, controlled attenuation parameter; SII, systemic immune-inflammation index.
In subgroup analyses stratified by gender, our results suggest that the positive association between SII and CAP is independently significantly positive in men [0.011 (0.004, 0.018)] but not statistically significant in all models for women. When we performed a subgroup analysis stratified by the degree of hepatic steatosis, the SII showed a strong positive correlation with both the NAFLD group and the severe steatosis group in both the unadjusted and partially adjusted models using the non-NAFLD group as the reference group.
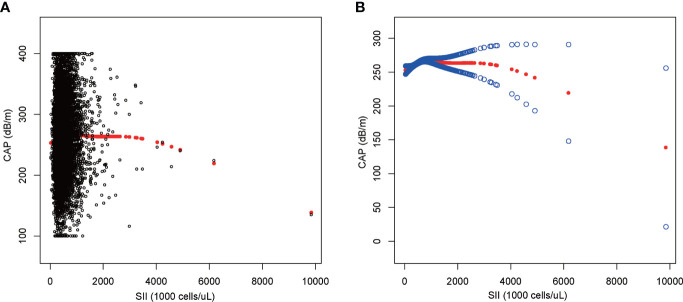
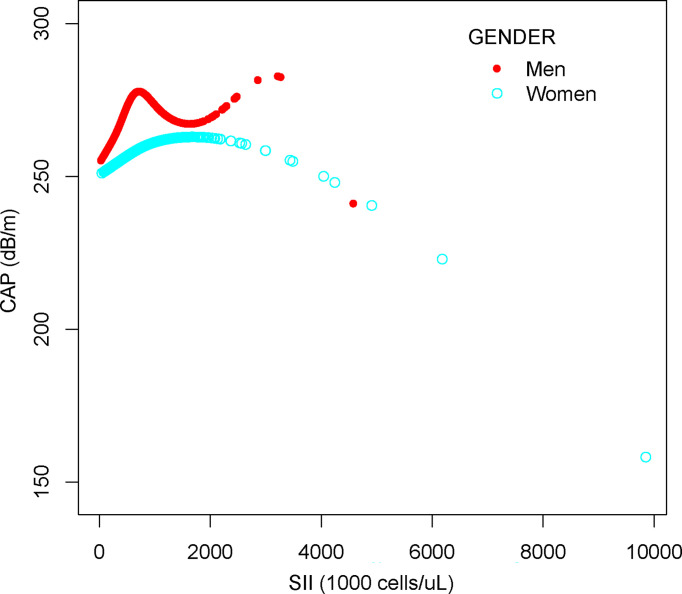
We performed a smooth curve fit to describe the nonlinear relationship between SII and CAP ( Figures 2 , 3 ). Using a two-segment linear regression model, we found an inverted U-shaped relationship between SII and CAP with an inflection point of 687.059 (1,000 cells/µl). After stratifying the analysis by gender, an inverted U-shaped curve was also present in men and women, with inflection points of 591.000 (1,000 cells/µl) and 749.692 (1,000 cells/µl), respectively ( Table 4 ).
Figure 2.
The association between SII and CAP. (A) Each black point represents a sample. (B) The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. SII, systemic immune-inflammation index; CAP, controlled attenuation parameter.
Figure 3.
The association between SII and CAP stratified by gender. SII, systemic immune-inflammation index; CAP, controlled attenuation parameter.
Table 4.
Threshold effect analysis of SII on CAP using two-piecewise linear regression model.
| CAP (dB/m) | Adjusted β (95% CI)P value |
|---|---|
| SII | |
| Inflection point | 687.059 |
| SII<687.059 (1,000 cells/µl) | 0.026 (0.017, 0.035) <0.0001 |
| SII>687.059 (1,000 cells/µl) | -0.008 (-0.015, -0.002) 0.0107 |
| Log likelihood ratio | <0.001 |
| Men | |
| Inflection point | 591.000 |
| SII<591.000 (1,000 cells/µl) | 0.045 (0.029, 0.062) <0.0001 |
| SII>591.000 (1,000 cells/µl) | -0.008 (-0.019, 0.003) 0.1556 |
| Log likelihood ratio | <0.001 |
| Women | |
| Inflection point | 749.692 |
| SII<749.692 (1,000 cells/µl) | 0.022 (0.011, 0.033) 0.0001 |
| SII>749.692 (1,000 cells/µl) | -0.008 (-0.016, -0.000) 0.0372 |
| Log likelihood ratio | <0.001 |
Age, gender, race, educational level, BMI, family income-to-poverty ratio, moderate activities, smoking status, ALP, ALT, AST, total calcium, total cholesterol, triglyceride, LDL, HDL-C, waist circumference, and serum phosphorus were adjusted.
LSM, liver stiffness measure ; SII, systemic immune-inflammation index.
Association between SII and LSM
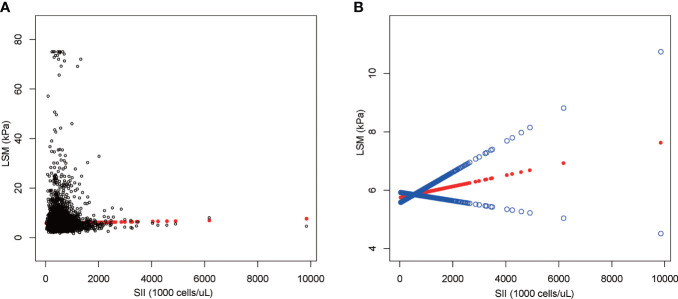
The results of multiple regression analysis showed a positive but insignificant correlation between SII and LSM ( Table 5 ). Moreover, the effect value was shown to be zero within three decimal places because the units of SII were too small [0.000 (-0.000, 0.001)]. Among all subgroup analyses, SII showed a statistically significant negative correlation with LSM only in the significant fibrosis group [-0.000 (-0.000, -0.000), P = 0.044]. The nonlinear relationship was characterized by smooth curve fittings ( Figure 4 ).
Table 5.
The association between SII and LSM.
| Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | |
|---|---|---|---|
| LSM (kPa) | 0.000 (-0.000, 0.001) | 0.000 (-0.000, 0.001) | -0.000 (-0.001, 0.001) |
| 0.263 | 0.182 | 0.927 | |
| Stratified by LSM | |||
| Normal group | Reference | Reference | Reference |
| Significant fibrosis | -0.000 (-0.000, -0.000) | -0.000 (-0.000, -0.000) | -0.000 (-0.000, -0.000) |
| 0.044 | 0.040 | 0.513 | |
| Advanced fibrosis | -0.000 (-0.000, -0.000) | -0.000 (-0.000, -0.000) | 0.000 (-0.000, 0.001) |
| 0.920 | 0.967 | 0.357 | |
| Cirrhosis | -0.001 (-0.008, 0.006) | -0.000 (-0.008, 0.007) | 0.000 (-0.013, 0.013) |
| 0.813 | 0.924 | 0.967 | |
| Stratified by gender | |||
| Men | 0.000 (-0.000, 0.001) | 0.000 (-0.001, 0.001) | -0.000 (-0.001, 0.001) |
| 0.432 | 0.867 | 0.872 | |
| Women | 0.000 (-0.000, 0.001) | 0.000 (-0.001, 0.001) | -0.000 (-0.001, 0.001) |
| 0.145 | 0.095 | 0.970 | |
Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, educational level, BMI, family income-to-poverty ratio, moderate activities, smoking status, ALP, ALT, AST, total calcium, total cholesterol, triglyceride, LDL, HDL-C, waist circumference, and serum phosphorus were adjusted.
In the subgroup analysis stratified by gender and race, the model is not adjusted for sex and race, respectively.
CAP, controlled attenuation parameter; SII, systemic immune-inflammation index.
Figure 4.
The association between SII and LSM. (A) Each black point represents a sample. (B) The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. SII, systemic immune-inflammation index; LSM, liver stiffness measurement.
Discussion
In our study sample, which is nationally representative of US adults, SII levels were positively correlated with hepatic steatosis and there was no significant correlation between SII levels and liver fibrosis. Notably, we found an inverted U-shaped association between SII and CAP, with an inflection point of 687.059 (1,000 cells/µl). This indicated that SII was an independent crisis factor for hepatic steatosis when the SII was less than 687.059 (1,000 cells/µl).
To our knowledge, this is the first study to investigate SII with hepatic steatosis and fibrosis. In previous studies on the liver, SII has often been used as a predictor of prognostic survival in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma (ICC) (38–40). Ren et al. (41) reported that among 28 patients with ICC who received liver transplantation, the 1-, 3-, and 5-year survival rates were significantly lower in the high-SII group than those in the low-SII group, and that SII could be used to predict survival in patients with ICC who received liver transplantation. Similarly, another study from China showed that SII was a valid prognostic factor for predicting the prognosis of patients undergoing radical hepatectomy for ICC, while neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) were not associated with clinical outcomes in these patients (42).
At present, many epidemiological studies have proven that inflammation is related to the progression of NAFLD (43–45). A large multicenter cohort of NAFLD patients from Italy and Finland showed that steatosis, ballooning, and lobular inflammation were independently associated with significant fibrosis. In addition, the authors found that a third of patients with significant fibrosis did not have non-alcoholic steatohepatitis (NASH) when they analyzed biopsy specimens taken from NAFLD patients at a single time point, a result that far exceeded expectations (46). The lack of significant association between SII and LSM found in our results may explain this phenomenon. Haukeland et al. (28) evaluated serum samples from 47 histologically validated NAFLD patients and showed that NAFLD patients are characterized by low-grade systemic inflammation. High chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemoattractant protein 1 (MCP1) levels in NASH may be important for the transition from simple steatosis to NASH (28). Our results demonstrate a significant positive relationship between SII and CAP; in other words, inflammation has a strong positive correlation with hepatic steatosis. Not only that, but the positive association between SII and CAP differs significantly by gender. Men with NAFLD have more severe hepatic steatosis than women, and postmenopausal women have greater hepatic steatosis than premenopausal women, according to several studies, suggesting that the gender difference in NAFLD is related to sex hormones (47, 48). Furthermore, a recent experimental animal study found that Formyl Peptide Receptor 2 (FPR2) expression is higher in female mice than that in male mice, making females more resistant to the development and progression of NAFLD, and the severe damage seen in FPR2-depleted females supports FPR2’s protective role in female mice’s liver (49). In addition to sex, race, age, and other covariates may also be factors influencing the relationship between SII and CAP, and multiple factors interacting with each other may also be the reason why the relationship between SII and CAP in this study was not significant in model 2 and model 3.
NAFLD includes a disease continuum from steatosis with or without mild inflammation to NASH, characterized by necrotizing inflammation and faster fibrotic progression than NAFLD (50). The mechanisms behind the connection between inflammation and NAFLD progression are unclear. One theory is that nutrient overload is the primary cause of NAFLD, with excess visceral fat causing macrophage infiltration into tissue compartments, resulting in a pro-inflammatory state that increases insulin resistance. Inappropriate lipolysis in the presence of insulin resistance causes aberrant fatty acid transport to the liver, resulting in a decrease in metabolic capacity. Lipotoxic lipids are formed as a result of lipid metabolic imbalances, which cause cellular stress, inflammasome activation, and apoptotic cell death, as well as stimulation of inflammation, tissue regeneration, and fibrogenesis (51, 52). This may be the mechanism leading to the progression of hepatic steatosis and fibrosis (53). Another theory is that metabolic imbalance and inflammation in NAFLD are caused by the liver’s interdependence and interaction with other organs (54–56). For example, differences in gut microbiota composition have been observed in NAFLD patients compared to the general population, and some data suggest the presence of fecal microbiome signatures associated with advanced fibrosis (57). Furthermore, substances produced by bacteria or bile acid metabolism can influence liver inflammation and disease progression in NAFLD, although a clear causal relationship has not been established (50, 57).
Our study has some limitations. First, this is a cross-sectional analysis; thus, temporality cannot be ascertained. Furthermore, despite adjusting several relevant confounders, we were unable to rule out the impact of additional confounding factors; therefore, our findings should be interpreted with caution. Third, due to the limitations of the NHANES database, the covariates of this study did not include participants’ medications use, and anti-inflammatory medications are often used in patients with NAFLD; therefore, our findings may not fully reflect the true situation. Fourth, the degree of hepatic steatosis and liver fibrosis in this study was judged by transient elastography, and although several studies have demonstrated the extremely high accuracy of transient elastography (58–60), it still cannot be the same as biopsy; therefore, our results may not be the same as using biopsy as a judgment of hepatic steatosis and liver fibrosis. Despite these limitations, our study has several advantages. Because we used a nationally representative sample, our study is representative of a multiethnic and gender-diverse population of adults in the United States. In addition to this, the large sample size included in our study allowed us to perform a subgroup analysis.
Conclusion
Our findings imply that increased SII levels are linked to hepatic steatosis, but SII is not linked to liver fibrosis. To confirm our findings, more large-scale prospective investigations are needed.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.
Ethics statement
This study was reviewed and approved by NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
RX and YZ designed the research. RX, YZ, MX and LL collected and analyzed the data. RX, XH and YZ drafted the manuscript. ML, NM and RX revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.925690/full#supplementary-material
Abbreviations
NAFLD, non-alcoholic fatty liver disease; CAP, controlled attenuation parameter; LSM, liver stiffness measurement; SII, systemic immune-inflammation index; NHANES, National Health and Nutrition Examination Survey; ICC, intrahepatic cholangiocarcinoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; NASH, non-alcoholic steatohepatitis.
References
- 1. Díaz LA, Fuentes-López E, Ayares G, Idalsoaga F, Arnold J, Márquez-Lomas A, et al. The establishment of public health policies and the burden of non-alcoholic fatty liver disease in the americas. Lancet Gastroenterol Hepatol (2022) 7(6):552–9. doi: 10.1016/S2468-1253(22)00008-5 [DOI] [PubMed] [Google Scholar]
- 2. Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X [DOI] [PubMed] [Google Scholar]
- 3. Rich NE, Noureddin M, Kanwal F, Singal AG. Racial and ethnic disparities in non-alcoholic fatty liver disease in the USA. Lancet Gastroenterol Hepatol (2021) 6:422–4. doi: 10.1016/S2468-1253(21)00100-X [DOI] [PubMed] [Google Scholar]
- 4. Xie R, Liu M. Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol (2022) 13. doi: 10.3389/fendo.2022.857110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol (1999) 94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x [DOI] [PubMed] [Google Scholar]
- 6. Long MT, Zhang X, Xu H, Liu CT, Corey KE, Chung RT, et al. Hepatic fibrosis associates with multiple cardiometabolic disease risk factors: The framingham heart study. Hepatology (2021) 73:548–59. doi: 10.1002/hep.31608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology (2019) 156:1264–1281.e4. doi: 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boursier J, Tsochatzis EA. Case-finding strategies in non-alcoholic fatty liver disease. JHEP Rep (2021) 3:100219. doi: 10.1016/j.jhepr.2020.100219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eddowes P, Sasso M, Allison M, Tsochatzis E, Anstee Q, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 10. Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care (2021) 44:519–25. doi: 10.2337/dc20-1778 [DOI] [PubMed] [Google Scholar]
- 11. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Zhou D, Dai Z, Li X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging (2021) 16:97–105. doi: 10.2147/CIA.S285000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol (2017) 23:6261–72. doi: 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang F, Niu M, Wang L, Liu Y, Shi L, Cao J, et al. Systemic-Immune-Inflammation index as a promising biomarker for predicting perioperative ischemic stroke in older patients who underwent non-cardiac surgery. Front Aging Neurosci (2022) 14:865244. doi: 10.3389/fnagi.2022.865244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian T, Lu J, Zhao W, Wang Z, Xu H, Ding Y, et al. Associations of systemic inflammation markers with identification of pulmonary nodule and incident lung cancer in Chinese population. Cancer Med (2022) 11:2482–91. doi: 10.1002/cam4.4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu YY, Ruan GT, Ge YZ, Li QQ, Zhang Q, Zhang X, et al. Systemic inflammation with sarcopenia predicts survival in patients with gastric cancer. J Cancer Res Clin Oncol (2022) 148:3925–6. doi: 10.1007/s00432-022-03925-2 [DOI] [PubMed] [Google Scholar]
- 17. He K, Si L, Pan X, Sun L, Wang Y, Lu J, et al. Preoperative systemic immune-inflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol (2022) 12:829689. doi: 10.3389/fonc.2022.829689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Feng H, Zhang W, Wei F, Zhou L, Liu L, et al. Prediction of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint inhibitors based on clinical and hematological markers: Real-world evidence. Exp Cell Res (2022) 416(1):113157. doi: 10.1016/j.yexcr.2022.113157 [DOI] [PubMed] [Google Scholar]
- 19. Yilmaz M, Baran A, Yilmaz MK. Predictive significance of inflammatory indexes in metastatic nonsmall cell lung cancer patients treated with platinum-doublet chemotherapy. J Cancer Res Ther (2022) 18:220–3. doi: 10.4103/jcrt.jcrt_1902_20 [DOI] [PubMed] [Google Scholar]
- 20. Aziz MH, van Dongen JC, Saida L, Suker M, van Vugt JLA, van Putten Y, et al. High systemic immune inflammation index is associated with low skeletal muscle quantity in resectable pancreatic ductal adenocarcinoma. Front Oncol (2022) 12:827755. doi: 10.3389/fonc.2022.827755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han R, Tian Z, Jiang Y, Guan G, Sun X, Yu Y, et al. Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery. Gland Surg (2022) 11:576–87. doi: 10.21037/gs-22-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang Z, Zheng Q, Yu Y, Zheng H, Wu Y, Wang Z, et al. Prognostic significance of platelet-to-albumin ratio in patients with esophageal squamous cell carcinoma receiving definitive radiotherapy. Sci Rep (2022) 12:3535. doi: 10.1038/s41598-022-07546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yaşar E, Bayramoğlu A. Systemic immune-inflammation index as a predictor of microvascular dysfunction in patients with cardiac syndrome X. Angiology (2022) 73:615–21. doi: 10.1177/00033197221087777 [DOI] [PubMed] [Google Scholar]
- 24. Zhou YX, Li WC, Xia SH, Xiang T, Tang C, Luo JL, et al. Predictive value of the systemic immune inflammation index for adverse outcomes in patients with acute ischemic stroke. Front Neurol (2022) 13:836595. doi: 10.3389/fneur.2022.836595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yavuz S, Engin M. Atherosclerotic burden and systemic immune inflammation index. Echocardiography (2022) 39:654. doi: 10.1111/echo.15343 [DOI] [PubMed] [Google Scholar]
- 26. Orhan AL, Şaylık F, Çiçek V, Akbulut T, Selçuk M, Çınar T. Evaluating the systemic immune-inflammation index for in-hospital and long-term mortality in elderly non-ST-elevation myocardial infarction patients. Aging Clin Exp Res (2022) 34:1687–95. doi: 10.1007/s40520-022-02103-1 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Liu W, Yu H, Chen Z, Zhang C, Ti Y, et al. Value of the systemic immune-inflammatory index (SII) in predicting the prognosis of patients with peripartum cardiomyopathy. Front Cardiovasc Med (2022) 9:811079. doi: 10.3389/fcvm.2022.811079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol (2006) 44:1167–74. doi: 10.1016/j.jhep.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 29. Rabelo F, Oliveira CP, Faintuch J, Mazo DF, Lima VM, Stefano JT, et al. Pro- and anti-inflammatory cytokines in steatosis and steatohepatitis. Obes Surg (2010) 20:906–12. doi: 10.1007/s11695-010-0181-4 [DOI] [PubMed] [Google Scholar]
- 30. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci (2016) 61:1294–303. doi: 10.1007/s10620-016-4049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. den Boer MA, Voshol PJ, Schröder-van der Elst JP, Korsheninnikova E, Ouwens DM, Kuipers F, et al. Endogenous interleukin-10 protects against hepatic steatosis but does not improve insulin sensitivity during high-fat feeding in mice. Endocrinology (2006) 147:4553–8. doi: 10.1210/en.2006-0417 [DOI] [PubMed] [Google Scholar]
- 32. Fontes-Cal TCM, Mattos RT, Medeiros NI, Pinto BF, Belchior-Bezerra M, Roque-Souza B, et al. Crosstalk between plasma cytokines, inflammation, and liver damage as a new strategy to monitoring NAFLD progression. Front Immunol (2021) 12:708959. doi: 10.3389/fimmu.2021.708959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, et al. The national health and nutrition examination survey: Sample design, 1999-2006. Vital Health Stat (2012) 2:1–39. [PubMed] [Google Scholar]
- 34. Albany C. Systemic immune-inflammation index in germ-cell tumours: Search for a biological prognostic biomarker. Br J Cancer (2018) 118:761–2. doi: 10.1038/bjc.2018.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang N, Lu Y, Cao L, Lu M. The association between non-alcoholic fatty liver disease and serum ferritin levels in American adults. J Clin Lab Anal (2022) 36:e24225. doi: 10.1002/jcla.24225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metab: Clin Exp (2021) 121:154752. doi: 10.1016/j.metabol.2021.154752 [DOI] [PubMed] [Google Scholar]
- 37. Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: Influence of gender and metabolic syndrome. J Hepatol (2008) 48:606–13. doi: 10.1016/j.jhep.2007.11.020 [DOI] [PubMed] [Google Scholar]
- 38. Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg (2021) 25:421–7. doi: 10.1007/s11605-019-04492-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu Y, Xin D, Wang F. Predictive significance of preoperative systemic immune-inflammation index determination in postoperative liver metastasis of colorectal cancer. Onco Targets Ther (2019) 12:7791–9. doi: 10.2147/OTT.S223419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu H, Zheng J, Cai J, Zeng K, Yao J, Chen L, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within hangzhou criteria. Cell Physiol Biochem (2018) 47:293–301. doi: 10.1159/000489807 [DOI] [PubMed] [Google Scholar]
- 41. Ren A, Li Z, Cheng P, Zhang X, Deng R, Ma Y. Systemic immune-inflammation index is a prognostic predictor in patients with intrahepatic cholangiocarcinoma undergoing liver transplantation. Mediators Inflammation (2021) 2021:6656996. doi: 10.1155/2021/6656996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Z, Zhou Y, Hu K, Huang Y. Investigating effects of preoperative inflammatory biomarkers on predicting survival outcomes of intrahepatic cholangiocarcinoma after curative resection. World J Surg Oncol (2020) 18:272. doi: 10.1186/s12957-020-02053-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur J Sport Sci (2019) 19:994–1003. doi: 10.1080/17461391.2019.1571114 [DOI] [PubMed] [Google Scholar]
- 44. de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol (2019) 70:710–21. doi: 10.1016/j.jhep.2018.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun (2018) 9:4462. doi: 10.1038/s41467-018-06929-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pelusi S, Cespiati A, Rametta R, Pennisi G, Mannisto V, Rosso C, et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without steatohepatitis. Clin Gastroenterol Hepatol (2019) 17:2310–2319.e6. doi: 10.1016/j.cgh.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 47. Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol (2006) 21:138–43. doi: 10.1111/j.1440-1746.2005.04086.x [DOI] [PubMed] [Google Scholar]
- 48. Suzuki A, Angulo P, Lymp J, St Sauver J, Muto A, Okada T, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology (2005) 41:64–71. doi: 10.1002/hep.20543 [DOI] [PubMed] [Google Scholar]
- 49. Lee C, Kim J, Han J, Oh D, Kim M, Jeong H, et al. Formyl peptide receptor 2 determines sex-specific differences in the progression of nonalcoholic fatty liver disease and steatohepatitis. Nat Commun (2022) 13:578. doi: 10.1038/s41467-022-28138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet (2021) 397:2212–24. doi: 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 51. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol (2019) 16:377–86. doi: 10.1038/s41575-019-0144-8 [DOI] [PubMed] [Google Scholar]
- 53. Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep (2019) 1:30–43. doi: 10.1016/j.jhepr.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature (2018) 555:673–7. doi: 10.1038/nature26138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Azzu V, Vacca M, Virtue S, Allison M, Vidal-Puig A. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology (2020) 158:1899–912. doi: 10.1053/j.gastro.2019.12.054 [DOI] [PubMed] [Google Scholar]
- 56. Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Nonalcoholic fatty liver disease: Modulating gut microbiota to improve severity? Gastroenterology (2020) 158:1881–98. doi: 10.1053/j.gastro.2020.01.049 [DOI] [PubMed] [Google Scholar]
- 57. Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab (2019) 30:607. doi: 10.1016/j.cmet.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shen M, Lee A, Lefkowitch JH, Worman HJ. Vibration-controlled transient elastography for assessment of liver fibrosis at a USA academic medical center. J Clin Transl Hepatol (2022) 10:197–206. doi: 10.14218/JCTH.2021.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abrams GA, Ware D, Byrne MM, Hecht EM. Risk stratification of adolescents for the screening of non-alcoholic fatty liver disease. Pediatr Obes (2022) 17:12924. doi: 10.1111/ijpo.12924 [DOI] [PubMed] [Google Scholar]
- 60. Argalia G, Ventura C, Tosi N, Campioni D, Tagliati C, Tufillaro M, et al. Comparison of point shear wave elastography and transient elastography in the evaluation of patients with NAFLD. Radiol Med (2022) 127:571–6. doi: 10.1007/s11547-022-01475-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.