Abstract

To improve perovskite solar cell (PSC) performance, which is deeply related to perovskite layer quality, researchers have explored numerous strategies. Additive doping into perovskite precursors has been widely used to improve the PSC performance. In this study, we used isoxazole—a Lewis-base small molecule—as an additive for the CH3NH3PbI3 (MAPbI3) precursor and explored how isoxazole effectively passivates defects in the perovskite structure. We found that isoxazole interacted with undercoordinated Pb2+ ions from an X-ray photoelectron spectroscopy survey and verified that isoxazole doping improved the device performance. When the optimized concentration of isoxazole was doped in the MAPbI3 precursor, the power conversion efficiency increased from 15.6 to 17.5%, with an improved fill factor and short-circuit current density. In addition, an isoxazole-doped device sustained 94% of its initial performance after 8 days under ambient air conditions (10 ± 5 RH %, 25 °C), whereas a device without isoxazole doping only maintained 64% of its initial performance.
1. Introduction
To solve the fuel energy problems, solar energy has been considered an alternative future energy source.1 Perovskite solar cells (PSCs) have been widely researched for solar energy generation applications due to advantages such as cost-effective fabrication,2 easy fabrication processes,3 tunable band gaps,4 long carrier lifetimes,5 and high absorption coefficients.6 In particular, the inorganic–organic hybrid PSCs based on CH3NH3PbI3 (MAPbI3) have been studied extensively.7−16 The power conversion efficiency (PCE) of PSCs has increased tremendously from 3.9 to 25.7% over the past 11 years.17,18 Furthermore, an inverted PSC with the p–i–n structure has attracted considerable attention due to its metal oxide-free layer and low processing temperature.19
Meanwhile, defects in perovskites are responsible for nonradiative recombination,20 and thus, their passivation has attracted much attention for use in suppressing defect-induced PSC degradation. Many researchers have explored defect passivation through molecular interactions between defects and various functional groups.15,21−26 Particularly, Lewis-base molecules contain N,27−29 S,27,28,30−32 O,33−35 and P36,37 and hence can donate nonbonding electrons, reacting with and passivating undercoordinated Pb2+ ions or Pb clusters, which act as a nonradiative recombination center and induce perovskite phase degradation by reacting with O2 and H2O.38 Compared with large Lewis-base molecules, small Lewis-base molecules might easily diffuse into the interior of bulk perovskites and hence are efficient for bulk defect passivation, whereas they can easily escape from the crystal, unable to passivate defects. Therefore, it is crucial to discover small molecules that improve the performance of PSCs. Previous studies have reported that poly(1-vinyl-3-ethyl-acetate) imidazole tetrafluoroborate,39 bithiophene-based n-type conjugated small molecules,40 4-dimethylaminopyridine,41 and dicyandiamide42 can serve as small-molecule additives for PSCs to improve their performance.
In this study, we used isoxazole—a small Lewis-base molecule—as an additive to the perovskite precursor for improving the PSC performance. Isoxazole is a weak base heterocyclic compound containing both N and O atoms,43 whose molecular structure is shown in Figure S1, and it has been widely used in drug synthesis.44,45 We explored how this compound played a role in the perovskite structure and demonstrated that a small amount of isoxazole doping enhanced the PSC performance and reduced nonradiative recombination by the defect passivation. Doping 0.4 M isoxazole into the perovskite precursor improved the PCE of the best device from 15.63 to 17.50% with a great increase in JSC. In addition, the isoxazole-added device retained 94% of its initial PCE under ambient air conditions (10 ± 5 RH %, 25 °C) after 8 day storage, indicating that it had better stability than the control device without isoxazole doping, which retained only 64% of its initial PCE.
2. Experimental Section
2.1. Materials
Greatcell Solar Materials Pty Ltd. (Queanbeyan, Australia) produced methylammonium iodide (MAI). Bathocuproine (BCP) and lead(II) iodide (PbI2) were obtained from TCI (Tokyo, Japan). Indium tin oxide (ITO)-patterned glass was obtained from Zhuhai Kaivo Optoelectronic Technology Co., Ltd. (Zhuhai, China). Sigma-Aldrich (St. Louis, MO, United States) provided chlorobenzene (CB), isoxazole, N,N-dimethylformamide (DMF), poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA), dimethyl sulfoxide (DMSO), Hellmanex III for detergent, and isopropyl alcohol (IPA). Nano-C Inc. (Westwood, MA, United States) provided [6,6]-phenyl-C61-butyric acid methyl ester (PCBM). All these chemical materials were used without any purification.
2.2. Device Fabrication
About 1.2 M PbI2 (553 mg) and 1.2 M MAI (190 mg) were added to DMF (700 μL) and DMSO (300 μL) for the precursor preparation. We added 0.2, 0.4, or 0.6 M isoxazole into the precursor solution for the experimental sample preparation, whereas no isoxazole was added for the control sample. All precursors were stored for 12 h at room temperature. For the hole-transport layer, 1.5 mg of PTAA was dissolved in 1 mL of toluene. For the electron transport layer, PCBM (20 mg/mL in CB) and BCP (1.5 mg/mL in IPA) were heated for 2 h on a hot plate at 60 °C. For device fabrication, ITO-coated glass was cleansed by ultrasonic treatment sequentially with a detergent, deionized water, and IPA for 25 min each. After cleaning, the substrates were treated with ultraviolet (UV) ozone plasma for 30 min. We coated the PTAA layer on a substrate via a spin-coating process at 4000 rpm for 33 s (initial 3 s for acceleration), followed by a heating procedure at 120 °C for 10 min. The perovskite layer was deposited on the PTAA-coated substrate at 1000 rpm for 10 s and 5000 rpm for 35 s, for which acceleration times were 1 and 5 s, respectively. 15 s after the second acceleration, CB (350 μL) was dripped on the substrate. After depositing the active layer, we put the sample on a hot plate at 60 °C for 30 s and 90 °C for 20 min. The next step was spin-coating PCBM (60 μL) and BCP (60 μL) at 1500 rpm for 30 s and 4000 rpm for 30 s, respectively, with an acceleration time of 5 s. From the PTAA deposition to BCP fabrication, all steps were performed in a glove box filled with nitrogen gas. As the last step, Ag (100 nm) deposition on the electrode was conducted using a thermal evaporator under high-vacuum conditions (<8.0 × 10–6 Torr).
2.3. Characterization
We employed scanning electron microscopy (SEM) to observe the surface morphologies using JSM-7500F (JEOL, Tokyo, Japan). We used SmartLab (Rigaku, Tokyo, Japan) to study the X-ray diffraction (XRD) pattern of our MAPbI3-coated films. The Fourier transform infrared (FTIR) spectra were recorded using a Spectrum Two instrument (PerkinElmer, MA, United states) with a resolution of 2 cm–1. The current density–voltage (J–V) curve results and space-charge-limited current (SCLC) measurement were obtained using a Keithley 2400 source meter (Keithley, Cleveland, OH, United States) under illuminated and dark conditions. The standard 1 sun illumination condition (AM 1.5 G, 100 mW/cm2) was controlled using HAL-320 (Asahi spectra USA, Torrance, CA, United States). To probe the incident photon-to-current efficiency (IPCE), we employed Solar Cell Scan 100 (Zolix Instruments, Beijing, China). For X-ray photoelectron spectroscopy (XPS), we used AXIS-Nova (Kratos Inc., San Diego, CA, United States) to assess the binding energy of each perovskite film surface molecule. Time-resolved photoluminescence (TRPL) and photoluminescence (PL) measurements were obtained using Fluorolog3 (HORIBA, Kyoto, Japan); the excitation wavelength for PL and TRPL was 467 nm and the emission wavelength for TRPL was 750 nm. UV–visible (UV–vis) spectroscopy analysis was obtained using Ultra-3660 (Rigol, Beijing, China). Electrochemical impedance spectroscopy (EIS) was performed using VersaSTAT3 (Ametek Scientific Instruments, Berwyn, PA, United States) under dark-room conditions. All these characterizations and analyses were obtained under ambient air conditions without the capsulation process. To estimate the average grain size, we calculate the average area of the grain by dividing the entire SEM image area by the total number of grains, assuming that the grain shape is a perfect circle (in two dimensions), and regard the diameter as the lateral size.
3. Results and Discussion
Figure 1a–d shows top-view SEM images of perovskite films with different isoxazole concentrations. SEM results show that the control film and 0.2–0.6 M isoxazole-doped films showed similar film compactness. Notably, isoxazole-doped films had larger average grain sizes than the control film of 241 nm; that is, 310, 342, and 319 nm for 0.2, 0.4, and 0.6 M isoxazole-doping concentrations, respectively. Films filled with a larger grain have fewer grain boundaries, which may decrease the nonradiative recombination of carriers.46
Figure 1.
(a–d) Top-view SEM images of the MAPbI3 perovskite film while increasing the isoxazole-doping concentration from 0 to 0.6 M.
To explore the effect of isoxazole doping on the formation and crystallinity of perovskite phases, we measured the XRD patterns for perovskite films fabricated on ITO-patterned substrates. Figure 2a shows that all XRD patterns have two characteristic peaks of the perovskite structure at 14.10 and 28.42°, which are consistent with the (110) and (220) lattice planes, respectively.47 Besides, the isoxazole-coated films do not have any new characteristic peaks compared with the control film, indicating that the additive did not induce a new phase. The 0.4 M isoxazole-doped perovskite film has the highest peak intensity value at those peaks, indicating that it has the best crystallinity. As shown in Figure S1, isoxazole—the Lewis base—has N and O atoms and hence can react with and passivate the undercoordinated Pb clusters or Pb2+ ions on the surface, which behave as charge traps,48 by providing lone pair electrons. We performed XPS measurements to examine the perovskite film surface with and without isoxazole doping (Figure 2b). High-resolution XPS results of Pb 4f for the control film displayed two specific peaks at 137.90 and 142.78 eV, corresponding to Pb 4f7/2 and Pb 4f5/2,49,50 whereas the isoxazole-doped film had two peaks at 138.09 and 142.95 eV, respectively. Both peaks shift to higher binding energies by 0.19–0.17 eV, indicating that isoxazole anchored and reacted with Pb ions on the perovskite surface.51 In the 0.4 M isoxazole-doped sample, the intensities of characteristic peaks were lower than those in the control sample. This may be due to the fact that isoxazole molecules on the surface block some of the incident X-rays. However, as discussed above, XRD data indicated that the crystallinity of the perovskite film was best for the case with 0.4 M isoxazole, although some isoxazole molecules might be located at the film surface.
Figure 2.
(a) XRD patterns and (b) XPS data for Pb 4f7/2 and Pb 4f5/2 spectra of control and 0.4 M isoxazole-doped films. (c) FTIR spectra of isoxazole with and without PbI2.
To further verify the interaction between isoxazole and Pb2+ ions, we carried out FTIR spectroscopy measurements for 100.0 μL of isoxazole with and without 0.5 mg of PbI2. As shown in Figure 2c, the stretching vibrations of C=N, N–O, and C–O bonds were 1652, 1557, and 1218 cm–1 in pure isoxazole23,52,53 and were red-shifted to 1645, 1553, and 1213 cm–1 upon adding PbI2, respectively. The weakening of C=N, N–O, and C–O bonds might be due to the formation of coordinate bonds between N or O atoms in isoxazole and Pb2+ ions in PbI2, implying the attractive interaction between isoxazole and MAPbI3, similar to previous studies.54
To further investigate the isoxazole-doping effect, we measured
the UV–vis absorption spectra of the control and isoxazole-doped
perovskite films deposited on glass substrates (Figure 3a). Figure 3a shows that all films have almost the same absorption
edge at ca. 770 nm, and the Tauc plot in Figure 3b shows that the band gaps of films are almost
the same, that is, 1.56 eV, which is similar to the theoretically
calculated band gap of the MAPbI3 perovskite (1.5–1.6
eV).55 This indicates that isoxazole doping
slightly affects the band gap, which agrees with the SEM and XRD results,
where no noticeable changes upon isoxazole doping were observed in
the structure and morphology. The light absorption (Figure 3a,b) and steady-state PL (Figure 3c) for 0.4 M isoxazole
doping were the highest among the considered films, indicating that
optimized isoxazole doping can reduce defects and improve the crystallinity
of the perovskite56,57 and hence may enhance the PSC
performance. To further explore the isoxazole-doping effect on charge
carrier recombination dynamics, we performed the TRPL measurement
(Figure 3d). The TRPL
spectrum value can be obtained from a second-order exponential decay
formula:  , where A1 and A2 denote the decay amplitudes and τ1 and τ2 denote the fast and slow decay times,
respectively.58 The 0.4 M isoxazole-doped
film had a longer average PL decay time of 333.9 ns than the control
film (282.3 ns) (Table S1), indicating
that isoxazole doping can reduce the defects associated with nonradiative
recombination.
, where A1 and A2 denote the decay amplitudes and τ1 and τ2 denote the fast and slow decay times,
respectively.58 The 0.4 M isoxazole-doped
film had a longer average PL decay time of 333.9 ns than the control
film (282.3 ns) (Table S1), indicating
that isoxazole doping can reduce the defects associated with nonradiative
recombination.
Figure 3.
(a) UV–vis absorption spectra, (b) Tauc plots, and (c) PL spectra of perovskite films with increasing doping concentration. (d) TRPL spectra of the control and 0.4 M isoxazole-doped films.
We fabricated PSCs (Figure 4a) with a configuration of glass/ITO/PTAA/perovskite
with
or without isoxazole/PCBM/BCP/Ag with a 0.04 cm2 active
area to explore their photovoltaic performance. Figure S2 shows the cross-sectional SEM images of the device
without isoxazole doping (the control device) and the 0.4 M isoxazole-doped
device. The perovskite layer thicknesses of both devices are approximately
520 nm. Figure 4b shows
that the energy band of each layer is well aligned for electron and
hole transports, where the values of energy bands were obtained from
previous studies.59,60Figure 4c shows the J–V characteristic curve of the best device for each experimental
group; the 0.4 M isoxazole-doped device showed a photovoltaic performance
with a PCE of 17.5%, whereas the control device achieved such with
a PCE of 15.6%. The J–V curves
of all different concentrations are shown in Figure S3, and the details of the photovoltaic parameters are shown
in Table S2. The 0.4 M isoxazole-doped
device had a 1.4 mA/cm2 higher average short-circuit current
(JSC) than the control device, attributable
to the suppression of electrically active defects responsible for
nonradiative recombination through the reaction between isoxazole
and Pb ions.61Figure S4 and Table S3 show the hysteresis
results of each device and the detailed data under reverse scan (from Voc to 0 V) and forward scan (from 0 V to Voc). The hysteresis index (HI), which is given
by 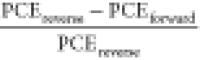 ,62 reduced from
0.04 to 0.02 using an isoxazole additive, where PCEreverse and PCEforward indicate the power conversion efficiencies
measured under reverse and forward bias conditions, respectively.
This is also explained by defect passivation by isoxazole.63,64 As shown in Figure S5 and Table S2, after adding isoxazole, the standard
deviation of PCE decreased, implying that using isoxazole is beneficial
for obtaining better device uniformity in mass production. The 0.4
M isoxazole-doped device showed the highest average PCE of (17.1%),
which was 1.9% higher than that (15.2%) of the control device. The
increases in JSC and fill factor (FF)
are mainly responsible for the PCE improvement, whereas the change
in VOC is negligible upon isoxazole doping.
The average PCE for 0.6 M isoxazole doping was 16.3%, which decreased
by 0.8% compared with that for the 0.4 M doping group. This is consistent
with smaller perovskite grain sizes and worse crystallinity for 0.6
M isoxazole doping than those for 0.4 M isoxazole doping (Figures 1 and 2a). As seen from Figure 4d, the 0.4 M isoxazole-doped device shows a higher
IPCE than the control device at wavelengths between 350 and 800 nm.
The JSC values obtained by integration
of IPCE are 20.5 and 18.4 mA/cm2 for the 0.4 M isoxazole-doped
and control devices, respectively. These values are similar to the
measured JSC in Figure 4c, within 1.4%, indicating the high reliability
of our results. EIS was performed under dark conditions to explore
the carrier transfer process. Figure 4e shows the corresponding Nyquist plot and equivalent
circuit that includes Rs and Rrec; Rs comprises the electrode
and contact resistances between each layer,65 and Rrec is the charge recombination
resistance.66Figure 4e and Table S4 show that the Rrec value (8369 Ω)
of the 0.4 M isoxazole-doped device is significantly larger than that
of the control device (5966 Ω), showing suppressed charge recombination
upon isoxazole doping. To further probe into the charge recombination
in the devices, we measured the light intensity dependence of VOC for each device. The VOC dependence on the light intensity (I) is
given by VOC = nkTln(I)/e + constant, where T, e, k, and n are
the absolute temperature, electron charge, Boltzmann constant, and
ideality factor, respectively.67,68 From the Shockley–Read–Hall
recombination kinetics, a steeper slope (n > 1)
denotes
a higher trap state density of a device.69Figure 4f shows that
the 0.4 M isoxazole-doped device has a lower slope (1.27 kT/e) than the control device (1.52 kT/e). The improved Rrec and the reduced n indicate that isoxazole doping
can passivate charge traps in PSCs.
,62 reduced from
0.04 to 0.02 using an isoxazole additive, where PCEreverse and PCEforward indicate the power conversion efficiencies
measured under reverse and forward bias conditions, respectively.
This is also explained by defect passivation by isoxazole.63,64 As shown in Figure S5 and Table S2, after adding isoxazole, the standard
deviation of PCE decreased, implying that using isoxazole is beneficial
for obtaining better device uniformity in mass production. The 0.4
M isoxazole-doped device showed the highest average PCE of (17.1%),
which was 1.9% higher than that (15.2%) of the control device. The
increases in JSC and fill factor (FF)
are mainly responsible for the PCE improvement, whereas the change
in VOC is negligible upon isoxazole doping.
The average PCE for 0.6 M isoxazole doping was 16.3%, which decreased
by 0.8% compared with that for the 0.4 M doping group. This is consistent
with smaller perovskite grain sizes and worse crystallinity for 0.6
M isoxazole doping than those for 0.4 M isoxazole doping (Figures 1 and 2a). As seen from Figure 4d, the 0.4 M isoxazole-doped device shows a higher
IPCE than the control device at wavelengths between 350 and 800 nm.
The JSC values obtained by integration
of IPCE are 20.5 and 18.4 mA/cm2 for the 0.4 M isoxazole-doped
and control devices, respectively. These values are similar to the
measured JSC in Figure 4c, within 1.4%, indicating the high reliability
of our results. EIS was performed under dark conditions to explore
the carrier transfer process. Figure 4e shows the corresponding Nyquist plot and equivalent
circuit that includes Rs and Rrec; Rs comprises the electrode
and contact resistances between each layer,65 and Rrec is the charge recombination
resistance.66Figure 4e and Table S4 show that the Rrec value (8369 Ω)
of the 0.4 M isoxazole-doped device is significantly larger than that
of the control device (5966 Ω), showing suppressed charge recombination
upon isoxazole doping. To further probe into the charge recombination
in the devices, we measured the light intensity dependence of VOC for each device. The VOC dependence on the light intensity (I) is
given by VOC = nkTln(I)/e + constant, where T, e, k, and n are
the absolute temperature, electron charge, Boltzmann constant, and
ideality factor, respectively.67,68 From the Shockley–Read–Hall
recombination kinetics, a steeper slope (n > 1)
denotes
a higher trap state density of a device.69Figure 4f shows that
the 0.4 M isoxazole-doped device has a lower slope (1.27 kT/e) than the control device (1.52 kT/e). The improved Rrec and the reduced n indicate that isoxazole doping
can passivate charge traps in PSCs.
Figure 4.
(a) Schematic structure and (b) energy band alignments of PSCs. (c) J–V reverse scan curves, (d) IPCE and the integrated current density (integrated J) from the IPCE measurement using the AM 1.5 G photon flux spectrum, (e) EIS measurements, and (f) VOC dependence on the light intensity of the control and 0.4 M isoxazole-doped devices.
We obtained the SCLC to measure the trap density
(ntrap) of each device comprising ITO/perovskites
with and
without the isoxazole/Ag structure under dark conditions. Figure 5a,b shows a linear
Ohmic-type response at low bias voltages; if this voltage is greater
than the kink point known as the trap-filled limit voltage (VTFL), JSC starts
to show a significantly higher slope increase with voltage. VTFL is given by VTFL = 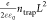 ,70 where ε,
ε0, e, ntrap, and L denote the relative dielectric constant,
vacuum permittivity, elementary charge, trap density, and thickness
of the perovskite, respectively; ε = 32.71 The thickness of the perovskite film was 520 nm, as discussed
above. The calculated value of the trap density of the 0.4 M isoxazole-doped
device was 1.40 × 1015 cm–3—lower
than that of the control device (1.76 × 1015 cm–3). This agrees with the results of crystallinity,
EIS, and light-intensity-dependent VOC measurements and indicates that the isoxazole additive passivates
defect-induced charge traps. Figure 5c shows the stabilized power output (SPO) and photocurrent
density (JSPO) at the maximum power point
voltage (Vmpp). The 0.4 M isoxazole-doped
device showed a more stable SPO of 16.9% and a JSPO of 19.7 mA/cm2 than those of the control device
(14.6% and 18.0 mA/cm2, respectively). We stored the devices
without capsulation in ambient air to investigate PCE stability. As
shown in Figure 5d,
the normalized PCE of the 0.4 M isoxazole-doped device remained over
94% of the initial PCE after 8 days, whereas the control device retained
only 64% of its initial PCE. It demonstrates that the isoxazole doping
passivated the defects such as unsaturated Pb ions, which have coordinate
bonds with O2 and H2O and hence cause perovskite
film degradation.38,72,73 The 0.4 M isoxazole-doped cell also showed the enhanced light soaking
stability under illumination (see Figure S6), which was measured by a method similar to that in previous studies.74,75 Our results indicate that isoxazole is an effective small-molecule
additive for improving the efficiency and stability of inverted PSCs
based on MAPbI3. Further study is required to prove that
isoxazole additives also improve the performances of different kinds
of PSCs.
,70 where ε,
ε0, e, ntrap, and L denote the relative dielectric constant,
vacuum permittivity, elementary charge, trap density, and thickness
of the perovskite, respectively; ε = 32.71 The thickness of the perovskite film was 520 nm, as discussed
above. The calculated value of the trap density of the 0.4 M isoxazole-doped
device was 1.40 × 1015 cm–3—lower
than that of the control device (1.76 × 1015 cm–3). This agrees with the results of crystallinity,
EIS, and light-intensity-dependent VOC measurements and indicates that the isoxazole additive passivates
defect-induced charge traps. Figure 5c shows the stabilized power output (SPO) and photocurrent
density (JSPO) at the maximum power point
voltage (Vmpp). The 0.4 M isoxazole-doped
device showed a more stable SPO of 16.9% and a JSPO of 19.7 mA/cm2 than those of the control device
(14.6% and 18.0 mA/cm2, respectively). We stored the devices
without capsulation in ambient air to investigate PCE stability. As
shown in Figure 5d,
the normalized PCE of the 0.4 M isoxazole-doped device remained over
94% of the initial PCE after 8 days, whereas the control device retained
only 64% of its initial PCE. It demonstrates that the isoxazole doping
passivated the defects such as unsaturated Pb ions, which have coordinate
bonds with O2 and H2O and hence cause perovskite
film degradation.38,72,73 The 0.4 M isoxazole-doped cell also showed the enhanced light soaking
stability under illumination (see Figure S6), which was measured by a method similar to that in previous studies.74,75 Our results indicate that isoxazole is an effective small-molecule
additive for improving the efficiency and stability of inverted PSCs
based on MAPbI3. Further study is required to prove that
isoxazole additives also improve the performances of different kinds
of PSCs.
Figure 5.
Dark J–V curves of the (a) control device and (b) 0.4 M isoxazole-doped device; (c) stabilized photocurrent densities and power output; and (d) stability measurement of the devices with and without isoxazole doping.
4. Conclusions
In summary, we improved the PCE of the optimal PSC from 15.6 to 17.5% via a small Lewis-base molecule isoxazole-doping technique. We found that isoxazole reacted with undercoordinated Pb2+ ion defects via XPS measurements, which agrees well with the reduction of electrically active charge traps by an isoxazole additive observed by TRPL, EIS, VOC versus light intensity, and SCLC measurements. Furthermore, the isoxazole additive increased the PSC stability under ambient air conditions. Our results indicate that isoxazole is one of the promising small-molecule additives that may effectively passivate defects inside and near interfaces.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2021R1F1A1051089) and the Gachon University Research Fund of 2020 (GCU-202008480006). The authors would like to thank Enago for the English language review.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03775.
Chemical structure of isoxazole; cross-sectional SEM image of the control and 0.4 M isoxazole-doped devices; J–V reverse scan curves for the control and isoxazole-doped devices; J–V forward scan curves for control and 0.4 M isoxazole-doped devices; statistics data of 15 devices with different isoxazole-doping concentrations; light soaking stability measurement under AM 1.5G illumination without encapsulation in air; detailed TRPL data of the control and 0.4 M isoxazole-doped films; device performance data for control and isoxazole-doped devices; and data of J–V curves of reverse and forward scan and HI; and detailed EIS data of perovskite devices with and without isoxazole (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kannan N.; Vakeesan D. Solar energy for future world: - A review. Renewable Sustainable Energy Rev. 2016, 62, 1092–1105. 10.1016/j.rser.2016.05.022. [DOI] [Google Scholar]
- Liu S.; Zhang D.; Sheng Y.; Zhang W.; Qin Z.; Qin M.; Li S.; Wang Y.; Gao C.; Wang Q.; Ming Y.; Liu C.; Yang K.; Huang Q.; Qi J.; Gao Q.; Chen K.; Hu Y.; Rong Y.; Lu X.; Mei A.; Han H. Highly oriented MAPbI3 crystals for efficient hole-conductor-free printable mesoscopic perovskite solar cells. Fundam. Res. 2022, 2, 276–283. 10.1016/j.fmre.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabi N.; Behjat A.; Zhou Y.; Docampo P.; Stoddard R. J.; Hillhouse H. W.; Ameri T. Progress and challenges in perovskite photovoltaics from single- to multi-junction cells. Mater. Today Energy 2019, 12, 70–94. 10.1016/j.mtener.2018.12.009. [DOI] [Google Scholar]
- Noh J. H.; Im S. H.; Heo J. H.; Mandal T. N.; Seok S. I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. 10.1021/nl400349b. [DOI] [PubMed] [Google Scholar]
- Zuo C.; Bolink H. J.; Han H.; Huang J.; Cahen D.; Ding L. Advances in Perovskite Solar Cells. Adv. Sci. 2016, 3, 1500324. 10.1002/advs.201500324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E. H.; Jeon N. J.; Park E. Y.; Moon C. S.; Shin T. J.; Yang T. Y.; Noh J. H.; Seo J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. 10.1038/s41586-019-1036-3. [DOI] [PubMed] [Google Scholar]
- Jeon N. J.; Noh J. H.; Kim Y. C.; Yang W. S.; Ryu S.; Seok S. I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. 10.1038/nmat4014. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Xie F.; Chen H.; Yang X.; Su H.; Cai M.; Zhou Z.; Noda T.; Han L. Thermally stable MAPbI3 perovskite solar cells with efficiency of 19.19% and area over 1 cm2 achieved by additive engineering. Adv. Mater. 2017, 29, 1701073. 10.1002/adma.201701073. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Turedi B.; Alsalloum A. Y.; Yang C.; Zheng X.; Gereige I.; AlSaggaf A.; Mohammed O. F.; Bakr O. M. Single-Crystal MAPbI3 Perovskite Solar Cells Exceeding 21% Power Conversion Efficiency. ACS Energy Lett. 2019, 4, 1258–1259. 10.1021/acsenergylett.9b00847. [DOI] [Google Scholar]
- Zhang Y.; Chen S.; Chen H.; Zhang G.; Zhao M.; Zhao C.; Guo W.; Ji W.; Shi Z.; Jiu T. Highly-improved performance of inverted planar perovskite solar cells by glucose modification. J. Mater. Chem. C 2020, 8, 5894–5903. 10.1039/d0tc00365d. [DOI] [Google Scholar]
- Abdelmageed G.; Jewell L.; Hellier K.; Seymour L.; Luo B.; Bridges F.; Zhang J. Z.; Carter S. Mechanisms for light induced degradation in MAPbI3 perovskite thin films and solar cells. Appl. Phys. Lett. 2016, 109, 233905. 10.1063/1.4967840. [DOI] [Google Scholar]
- Frolova L. A.; Dremova N. N.; Troshin P. A. The chemical origin of the p-type and n-type doping effects in the hybrid methylammonium-lead iodide (MAPbI3) perovskite solar cells. Chem. Commun. 2015, 51, 14917–14920. 10.1039/c5cc05205j. [DOI] [PubMed] [Google Scholar]
- Han Y.; Meyer S.; Dkhissi Y.; Weber K.; Pringle J. M.; Bach U.; Spiccia L.; Cheng Y.-B. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. 10.1039/c5ta00358j. [DOI] [Google Scholar]
- Cha M.; Da P.; Wang J.; Wang W.; Chen Z.; Xiu F.; Zheng G.; Wang Z. S. Enhancing Perovskite Solar Cell Performance by Interface Engineering Using CH3NH3PbBr0.9I2.1 Quantum Dots. J. Am. Chem. Soc. 2016, 138, 8581–8587. 10.1021/jacs.6b04519. [DOI] [PubMed] [Google Scholar]
- Ahn N.; Son D. Y.; Jang I. H.; Kang S. M.; Choi M.; Park N. G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. 10.1021/jacs.5b04930. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Seo J.; Park S.; Shin S. S.; Kim Y. C.; Jeon N. J.; Shin H. W.; Ahn T. K.; Noh J. H.; Yoon S. C.; Hwang C. S.; Seok S. I. Efficient CH3NH3PbI3Perovskite Solar Cells Employing Nanostructured p-Type NiO Electrode Formed by a Pulsed Laser Deposition. Adv. Mater. 2015, 27, 4013–4019. 10.1002/adma.201500523. [DOI] [PubMed] [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- National Renewable Energy Laboratory . Best Research Cell Efficiencies. 2022, https://www.nrel.gov/pv/cell-efficiency.html (accessed June 30, 2022).
- Said A. A.; Xie J.; Zhang Q. Recent Progress in Organic Electron Transport Materials in Inverted Perovskite Solar Cells. Small 2019, 15, 1900854. 10.1002/smll.201900854. [DOI] [PubMed] [Google Scholar]
- Chen J.; Park N. G. Causes and Solutions of Recombination in Perovskite Solar Cells. Adv. Mater. 2019, 31, 1803019. 10.1002/adma.201803019. [DOI] [PubMed] [Google Scholar]
- Lee D.-K.; Lim K.-S.; Lee J.-W.; Park N.-G. Scalable perovskite coating via anti-solvent-free Lewis acid–base adduct engineering for efficient perovskite solar modules. J. Mater. Chem. A 2021, 9, 3018–3028. 10.1039/d0ta10366g. [DOI] [Google Scholar]
- Manser J. S.; Christians J. A.; Kamat P. V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. 10.1021/acs.chemrev.6b00136. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Chen H.; Stoumpos C. C.; Ren J.; Hou Q.; Li X.; Li J.; He H.; Lin H.; Wang J.; Hao F.; Kanatzidis M. G. Thiazole-Induced Surface Passivation and Recrystallization of CH3NH3PbI3 Films for Perovskite Solar Cells with Ultrahigh Fill Factors. ACS Appl. Mater. Interfaces 2018, 10, 42436–42443. 10.1021/acsami.8b16124. [DOI] [PubMed] [Google Scholar]
- Liu X.; Wu J.; Guo Q.; Yang Y.; Luo H.; Liu Q.; Wang X.; He X.; Huang M.; Lan Z. Pyrrole: an additive for improving the efficiency and stability of perovskite solar cells. J. Mater. Chem. A 2019, 7, 11764–11770. 10.1039/c9ta02916h. [DOI] [Google Scholar]
- Deng L.; Xie S.; Gao F. Fullerene-Based Materials for Photovoltaic Applications: Toward Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells. Adv. Electron. Mater. 2018, 4, 1700435. 10.1002/aelm.201700435. [DOI] [Google Scholar]
- Jia L.; Chen M.; Yang S. Functionalization of fullerene materials toward applications in perovskite solar cells. Mater. Chem. Front. 2020, 4, 2256–2282. 10.1039/d0qm00295j. [DOI] [Google Scholar]
- Noel N. K.; Abate A.; Stranks S. D.; Parrott E. S.; Burlakov V. M.; Goriely A.; Snaith H. J. Enhanced photoluminescence and solar cell performance via Lewis base passivation of organic–inorganic lead halide perovskites. ACS Nano 2014, 8, 9815–9821. 10.1021/nn5036476. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Shen L.; Dai J.; Deng Y.; Wu Y.; Bai Y.; Zheng X.; Wang J.; Fang Y.; Wei H.; Ma W.; Zeng X. C.; Zhan X.; Huang J. π-conjugated Lewis Base: efficient trap-passivation and charge-extraction for hybrid perovskite solar cells. Adv. Mater. 2017, 29, 1604545. 10.1002/adma.201604545. [DOI] [PubMed] [Google Scholar]
- Zuo L.; Guo H.; deQuilettes D. W.; Jariwala S.; De Marco N.; Dong S.; DeBlock R.; Ginger D. S.; Dunn B.; Wang M.; Yang Y. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 2017, 3, e1700106 10.1126/sciadv.1700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Yin J.; Yuan S.; Zhao Y.; Li J.; Zheng N. Thiols as interfacial modifiers to enhance the performance and stability of perovskite solar cells. Nanoscale 2015, 7, 9443–9447. 10.1039/c5nr01820j. [DOI] [PubMed] [Google Scholar]
- Wen T. Y.; Yang S.; Liu P. F.; Tang L. J.; Qiao H. W.; Chen X.; Yang X. H.; Hou Y.; Yang H. G. Surface Electronic Modification of Perovskite Thin Film with Water-Resistant Electron Delocalized Molecules for Stable and Efficient Photovoltaics. Adv. Energy Mater. 2018, 8, 1703143. 10.1002/aenm.201703143. [DOI] [Google Scholar]
- Zeng Q.; Zhang X.; Feng X.; Lu S.; Chen Z.; Yong X.; Redfern S. A.; Wei H.; Wang H.; Shen H.; Zhang W.; Zheng W.; Zhang H.; Tse J. S.; Yang B. Polymer-passivated inorganic cesium lead mixed-halide perovskites for stable and efficient solar cells with high open-circuit voltage over 1.3 V. Adv. Mater. 2018, 30, 1705393. 10.1002/adma.201705393. [DOI] [PubMed] [Google Scholar]
- Eperon G. E.; Habisreutinger S. N.; Leijtens T.; Bruijnaers B. J.; van Franeker J. J.; deQuilettes D. W.; Pathak S.; Sutton R. J.; Grancini G.; Ginger D. S.; Janssen R. A. J.; Petrozza A.; Snaith H. J. The importance of moisture in hybrid lead halide perovskite thin film fabrication. ACS Nano 2015, 9, 9380–9393. 10.1021/acsnano.5b03626. [DOI] [PubMed] [Google Scholar]
- Li W.; Dong H.; Guo X.; Li N.; Li J.; Niu G.; Wang L. Graphene oxide as dual functional interface modifier for improving wettability and retarding recombination in hybrid perovskite solar cells. J. Mater. Chem. A 2014, 2, 20105–20111. 10.1039/c4ta05196c. [DOI] [Google Scholar]
- Palazon F.; Pérez-del-Rey D.; Marras S.; Prato M.; Sessolo M.; Bolink H. J.; Manna L. Coating Evaporated MAPI Thin Films with Organic Molecules: Improved Stability at High Temperature and Implementation in High-Efficiency Solar Cells. ACS Energy Lett. 2018, 3, 835–839. 10.1021/acsenergylett.8b00193. [DOI] [Google Scholar]
- deQuilettes D. W.; Koch S.; Burke S.; Paranji R. K.; Shropshire A. J.; Ziffer M. E.; Ginger D. S. Photoluminescence lifetimes exceeding 8 μs and quantum yields exceeding 30% in hybrid perovskite thin films by ligand passivation. ACS Energy Lett. 2016, 1, 438–444. 10.1021/acsenergylett.6b00236. [DOI] [Google Scholar]
- Braly I. L.; deQuilettes D. W.; Pazos-Outón L. M.; Burke S.; Ziffer M. E.; Ginger D. S.; Hillhouse H. W. Hybrid perovskite films approaching the radiative limit with over 90% photoluminescence quantum efficiency. Nat. Photonics 2018, 12, 355–361. 10.1038/s41566-018-0154-z. [DOI] [Google Scholar]
- Zhu J.; Kim D. H.; Kim J. D.; Lee D. G.; Kim W. B.; Chen S. w.; Kim J. Y.; Lee J. M.; Lee H.; Han G. S.; Ahn T. K.; Jung H. S. All-in-One Lewis Base for Enhanced Precursor and Device Stability in Highly Efficient Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 3425–3434. 10.1021/acsenergylett.1c01465. [DOI] [Google Scholar]
- Wang S.; Yang B.; Han J.; He Z.; Li T.; Cao Q.; Yang J.; Suo J.; Li X.; Liu Z.; Liu S.; Tang C.; Hagfeldt A. Polymeric room-temperature molten salt as a multifunctional additive toward highly efficient and stable inverted planar perovskite solar cells. Energy Environ. Sci. 2020, 13, 5068–5079. 10.1039/d0ee02043e. [DOI] [Google Scholar]
- Koo D.; Cho Y.; Kim U.; Jeong G.; Lee J.; Seo J.; Yang C.; Park H. High-performance inverted perovskite solar cells with operational stability via n-type small molecule additive-assisted defect passivation. Adv. Energy Mater. 2020, 10, 2001920. 10.1002/aenm.202001920. [DOI] [Google Scholar]
- Song S.; Park E. Y.; Ma B. S.; Kim D. J.; Park H. H.; Kim Y. Y.; Shin S. S.; Jeon N. J.; Kim T. S.; Seo J. Selective defect passivation and topographical control of 4-dimethylaminopyridine at grain boundary for efficient and stable planar perovskite solar cells. Adv. Energy Mater. 2021, 11, 2003382. 10.1002/aenm.202003382. [DOI] [Google Scholar]
- Han W.; Liu X.; Zhang X.; Ding Y.; Guo Y. Bifunctional-based small molecule as multifunctional additive for improved performance of perovskite solar cells. Org. Electron. 2021, 96, 106226. 10.1016/j.orgel.2021.106226. [DOI] [Google Scholar]
- Ram V. J.; Sethi A.; Nath M.; Pratap R.. Five-Membered Heterocycles. The Chemistry of Heterocycles; Elsevier, 2019; pp 149–478. [Google Scholar]
- Pairas G. N.; Perperopoulou F.; Tsoungas P. G.; Varvounis G. The Isoxazole Ring and Its N-Oxide: A Privileged Core Structure in Neuropsychiatric Therapeutics. ChemMedChem 2017, 12, 408–419. 10.1002/cmdc.201700023. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Mo J.; Lin H. Z.; Chen Y.; Sun H. P. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. 10.1016/j.bmc.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Sherkar T. S.; Momblona C.; Gil-Escrig L.; Ávila J.; Sessolo M.; Bolink H. J.; Koster L. J. A. Recombination in Perovskite Solar Cells: Significance of Grain Boundaries, Interface Traps, and Defect Ions. ACS Energy Lett. 2017, 2, 1214–1222. 10.1021/acsenergylett.7b00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson T. J.; Correa-Baena J. P.; Halvani Anaraki E.; Philippe B.; Stranks S. D.; Bouduban M. E.; Tress W.; Schenk K.; Teuscher J.; Moser J. E.; Rensmo H.; Hagfeldt A. Unreacted PbI2 as a Double-Edged Sword for Enhancing the Performance of Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 10331–10343. 10.1021/jacs.6b06320. [DOI] [PubMed] [Google Scholar]
- Chen B.; Rudd P. N.; Yang S.; Yuan Y.; Huang J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. 10.1039/c8cs00853a. [DOI] [PubMed] [Google Scholar]
- Xie H.; Liu X.; Lyu L.; Niu D.; Wang Q.; Huang J.; Gao Y. Effects of Precursor Ratios and Annealing on Electronic Structure and Surface Composition of CH3NH3PbI3 Perovskite Films. J. Phys. Chem. C 2015, 120, 215–220. 10.1021/acs.jpcc.5b07728. [DOI] [Google Scholar]
- Xu H.; Wu Y.; Cui J.; Ni C.; Xu F.; Cai J.; Hong F.; Fang Z.; Wang W.; Zhu J.; Wang L.; Xu R.; Xu F. Formation and evolution of the unexpected PbI2 phase at the interface during the growth of evaporated perovskite films. Phys. Chem. Chem. Phys. 2016, 18, 18607–18613. 10.1039/c6cp02737g. [DOI] [PubMed] [Google Scholar]
- Wang R.; Xue J.; Wang K.-L.; Wang Z.-K.; Luo Y.; Fenning D.; Xu G.; Nuryyeva S.; Huang T.; Zhao Y.; Yang J. L.; Zhu J.; Wang M.; Tan S.; Yavuz I.; Houk K. N.; Yang Y. Constructive molecular configurations for surface-defect passivation of perovskite photovoltaics. Science 2019, 366, 1509–1513. 10.1126/science.aay9698. [DOI] [PubMed] [Google Scholar]
- Ak M.; Gacal B.; Kiskan B.; Yagci Y.; Toppare L. Enhancing electrochromic properties of polypyrrole by silsesquioxane nanocages. Polymer 2008, 49, 2202–2210. 10.1016/j.polymer.2008.03.023. [DOI] [Google Scholar]
- Fu J.; Liu W.; Hao Z.; Wu J.; Yin A.; Panjiyar X.; Liu J.; Shen H.; Wang H. Characterization of a low shrinkage dental composite containing bismethylene spiroorthocarbonate expanding monomer. Int. J. Mol. Sci. 2014, 15, 2400–2412. 10.3390/ijms15022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi D.; Yi C.; Luo J.; Décoppet J. D.; Zhang F.; Zakeeruddin S. M.; Li X.; Hagfeldt A.; Grätzel M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21. Nat. Energy 2016, 1, 16142. 10.1038/nenergy.2016.142. [DOI] [Google Scholar]
- Leguy A. M.; Azarhoosh P.; Alonso M. I.; Campoy-Quiles M.; Weber O. J.; Yao J.; Bryant D.; Weller M. T.; Nelson J.; Walsh A.; van Schilfgaarde M.; Barnes P. R. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale 2016, 8, 6317–6327. 10.1039/c5nr05435d. [DOI] [PubMed] [Google Scholar]
- Wen X.; Wu J.; Gao D.; Lin C. Interfacial engineering with amino-functionalized graphene for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 13482–13487. 10.1039/c6ta04616a. [DOI] [Google Scholar]
- Li G.; Wu J.; Song J.; Meng C.; Song Z.; Wang X.; Liu X.; Yang Y.; Wang D.; Lan Z. Excellent quinoline additive in perovskite toward to efficient and stable perovskite solar cells. J. Power Sources 2021, 481, 228857. 10.1016/j.jpowsour.2020.228857. [DOI] [Google Scholar]
- Mohanta D.; Narayanan S. S.; Pal S. K.; Raychaudhuri A. K. Time-resolved photoluminescence decay characteristics of bovine serum albumin-conjugated semiconductor nanocrystallites. J. Exp. Nanosci. 2009, 4, 177–191. 10.1080/17458080902866204. [DOI] [Google Scholar]
- Chen C. I.; Wu S.; Lu Y. A.; Lee C. C.; Ho K. C.; Zhu Z.; Chen W. C.; Chueh C. C. Enhanced Near-Infrared Photoresponse of Inverted Perovskite Solar Cells Through Rational Design of Bulk-Heterojunction Electron-Transporting Layers. Adv. Sci. 2019, 6, 1901714. 10.1002/advs.201901714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.; Wu R.; Zhang Z.; Xiong J.; He Z.; Fan B.; Dai Z.; Yang B.; Xue X.; Cai P.; Zhan S.; Zhang X.; Zhang J. Achieving efficient inverted planar perovskite solar cells with nondoped PTAA as a hole transport layer. Org. Electron. 2019, 71, 106–112. 10.1016/j.orgel.2019.05.019. [DOI] [Google Scholar]
- Luo D.; Su R.; Zhang W.; Gong Q.; Zhu R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2019, 5, 44–60. 10.1038/s41578-019-0151-y. [DOI] [Google Scholar]
- Habisreutinger S. N.; Noel N. K.; Snaith H. J. Hysteresis Index: A Figure without Merit for Quantifying Hysteresis in Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2472–2476. 10.1021/acsenergylett.8b01627. [DOI] [Google Scholar]
- Hanmandlu C.; Swamy S.; Singh A.; Hsin-An Chen C.; Liu C.-C.; Lai C.-S.; Mohapatra A.; Pao C.-W.; Chen P.; Chu C.-W. Suppression of surface defects to achieve hysteresis-free inverted perovskite solar cells via quantum dot passivation. J. Mater. Chem. A 2020, 8, 5263–5274. 10.1039/c9ta12904a. [DOI] [Google Scholar]
- Miller D. W.; Eperon G. E.; Roe E. T.; Warren C. W.; Snaith H. J.; Lonergan M. C. Defect states in perovskite solar cells associated with hysteresis and performance. Appl. Phys. Lett. 2016, 109, 153902. 10.1063/1.4963760. [DOI] [Google Scholar]
- Tao L.; Huo Z.; Ding Y.; Li Y.; Dai S.; Wang L.; Zhu J.; Pan X.; Zhang B.; Yao J.; Nazeeruddin M. K.; Grätzel M. High-efficiency and stable quasi-solid-state dye-sensitized solar cell based on low molecular mass organogelator electrolyte. J. Mater. Chem. A 2015, 3, 2344–2352. 10.1039/c4ta06188h. [DOI] [Google Scholar]
- Jiang J.; Wang Q.; Jin Z.; Zhang X.; Lei J.; Bin H.; Zhang Z. G.; Li Y.; Liu S. Polymer doping for high-efficiency perovskite solar cells with improved moisture stability. Adv. Energy Mater. 2018, 8, 1701757. 10.1002/aenm.201701757. [DOI] [Google Scholar]
- Duan J.; Zhao Y.; He B.; Tang Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72 % Efficiency. Angew. Chem., Int. Ed. 2018, 57, 3787–3791. 10.1002/anie.201800019. [DOI] [PubMed] [Google Scholar]
- Li H.; Tong G.; Chen T.; Zhu H.; Li G.; Chang Y.; Wang L.; Jiang Y. Interface engineering using a perovskite derivative phase for efficient and stable CsPbBr3 solar cells. J. Mater. Chem. A 2018, 6, 14255–14261. 10.1039/c8ta03811b. [DOI] [Google Scholar]
- Yin P.; Zheng T.; Wu Y.; Liu G.; Zhang Z.-G.; Cui C.; Li Y.; Shen P. Achieving efficient thick active layer and large area ternary polymer solar cells by incorporating a new fused heptacyclic non-fullerene acceptor. J. Mater. Chem. A 2018, 6, 20313–20326. 10.1039/c8ta06836d. [DOI] [Google Scholar]
- Lampert M. A.; Mark P.. Current Injection in Solids; Academic press, 1970. [Google Scholar]
- Anusca I.; Balčiu̅nas S.; Gemeiner P.; Svirskas Š.; Sanlialp M.; Lackner G.; Fettkenhauer C.; Belovickis J.; Samulionis V.; Ivanov M.; Dkhil B.; Banys J.; Shvartsman V. V.; Lupascu D. C. Dielectric response: answer to many questions in the methylammonium lead halide solar cell absorbers. Adv. Energy Mater. 2017, 7, 1700600. 10.1002/aenm.201700600. [DOI] [Google Scholar]
- Senocrate A.; Acartürk T.; Kim G. Y.; Merkle R.; Starke U.; Grätzel M.; Maier J. Interaction of oxygen with halide perovskites. J. Mater. Chem. A 2018, 6, 10847–10855. 10.1039/c8ta04537b. [DOI] [Google Scholar]
- Huang J.; Tan S.; Lund P. D.; Zhou H. Impact of H2O on organic–inorganic hybrid perovskite solar cells. Energy Environ. Sci. 2017, 10, 2284–2311. 10.1039/c7ee01674c. [DOI] [Google Scholar]
- Cao J.; Loi H. L.; Xu Y.; Guo X.; Wang N.; Liu C. K.; Wang T.; Cheng H.; Zhu Y.; Li M. G.; Wong W. Y.; Yan F. High-Performance Tin-Lead Mixed-Perovskite Solar Cells with Vertical Compositioal Gradient. Adv. Mater. 2022, 34, 2107729. 10.1002/adma.202107729. [DOI] [PubMed] [Google Scholar]
- Deng X.; Wen X.; Zheng J.; Young T.; Lau C. F. J.; Kim J.; Green M.; Huang S.; Ho-Baillie A. Dynamic study of the light soaking effect on perovskite solar cells by in-situ photoluminescence microscopy. Nano Energy 2018, 46, 356–364. 10.1016/j.nanoen.2018.02.024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







