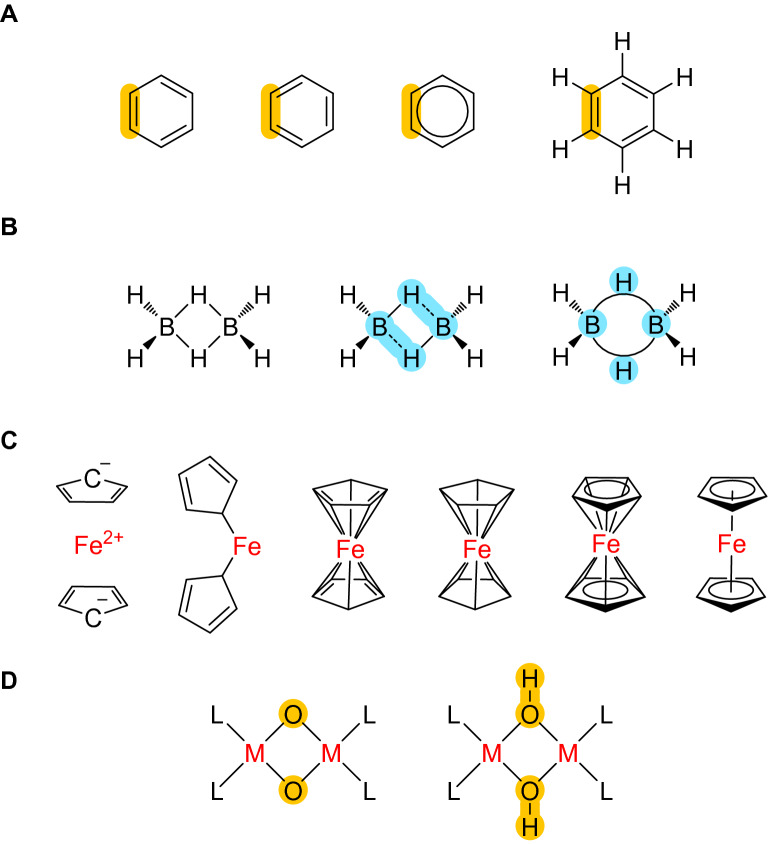
Fig. 1.
A Four different structural representations of benzene (C6H6) with one C–C bond highlighted in orange to serve as a reference, only the rightmost structure shows explicit hydrogens, B three different structural representations of diborane (B2H6), only the left structure preserves the correct symmetry but has an electron count that is too high by four electrons, the center structure tries to preserve the electron count by using “zero-order” bonds [2] indicated as dashed lines at the expense of an artificially lowered symmetry, and the right structure uses “banana bonds” to capture the three-center-two-electron (3c–2e) nature of the B–H–B bonding which however cannot be represented with traditional graph concepts, C different representations of ferrocene ([Fe(C5H5)2]), with only the 3rd to 5th from the left properly representing the 10 equal Fe–C bonds observed in X-ray structures, D Without explicit hydrogen atoms, it is impossible to distinguish between the bis-µ-oxo (left) and bis-µ-hydroxo (right) structures often encountered in inorganic chemistry, as there is no known algorithmic way to decide whether H atoms have to be added to the M2O2 core or not