Abstract
Purpose
One of the long-term symptoms of COVID-19 is phantosmia, a type of Olfactory Disorder (OD) that has deleterious impacts on patients’ quality of life. The aim of this article was to study how this poorly understood qualitative OD manifests itself in the COVID-19.
Methods
4691 patients with COVID-19 responded to our online questionnaire focusing on COVID-19-related OD. We first analyzed the prevalence of phantosmia in this population. Then, with the help of Natural Language Processing techniques, we investigated the qualitative descriptions of phantom smells by the 1723 respondents who reported phantosmia.
Results
The prevalence of phantosmia was of 37%. Women were more likely to report phantosmia than men, as well as respondents for whom OD was described as fluctuating rather than permanent, lasted longer, was partial rather than total and appeared progressively rather than suddenly. The relationship between OD duration and phantosmia followed a logarithmic function, with a prevalence of phantosmia increasing strongly during the first 2 months of the disease before reaching a plateau and no decrease over the 15 months considered in this study. Qualitative analyses of phantosmia descriptions with a sentiment analysis revealed that the descriptions were negatively valenced for 78% of the respondents. Reference to “tobacco” was more frequent in non-smokers. Source names and odor characteristics were used differently according to age and OD duration.
Conclusion
The results of this descriptive study of phantosmia contribute to the current efforts of the medical community to better understand and treat this rapidly increasing COVID-19-related OD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00405-022-07649-4.
Keywords: Olfaction, COVID-19, Phantosmia, Olfactory disorders, Quality of life, Self-report
Introduction
Quantitative Olfactory Disorders (ODs) are some of the earliest symptoms of COVID-19 [1, 2], as well as one of the longest lasting once acute symptoms of the disease have been cured [3, 4], inducing a reduction in the quality of life of patients [5]. Besides quantitative ODs, qualitative ones are also reported. These are parosmia (when olfactory sources smell differently than usual) and phantosmia: phantom smells are strange subjective experiences—olfactory hallucinations—occurring when no odor source is present in the environment [6]. These qualitative disorders often have an even more deleterious impact on daily life than the quantitative partial (i.e., hyposmia) or total (i.e., anosmia) loss of smell [6, 7], even though patients rarely seek medical support [8]. Research on COVID-19-induced OD reports that both parosmia and phantosmia become more prevalent as OD duration increases [9–11]. It has even been found that they can appear after a period of apparent recovery from COVID-19-induced OD [12].
Before the wake of the COVID-19 pandemic, research on phantosmia was not very advanced, potentially due to the subjective nature of this sensory phenomenon. However, coupled with research on other forms of sensory hallucinations, a few findings have emerged. First, the prevalence of phantosmia varies depending on the studies and the populations investigated (from 6.5% of the general population in , to 10% of Parkinson’s patients in , and 25.6% of patients with chemosensory and nasal/sinus complaints in ). This figure rises to 50% for patients suffering from head trauma or post-viral upper respiratory infections [14]. In COVID-19 patients, the prevalence of phantosmia also fluctuates between studies, from 10% [15] to 34% when OD is still present up to 11 months after the acute phase of the disease [11]. Second, the underlying mechanisms responsible for phantosmia and other sensory hallucinations are far from being understood, but typically involve peripheral and central causes [6, 16]. Third, phantom smells vary widely in their forms from subject to subject [16]: they may have different durations and frequencies of occurrence, and may be associated or not with other ODs. They are also more often reported by women than by men [8, 16], but not always [10]. Besides, contrary to quantitative ODs that increase with age, experiencing phantom smells was found to be not affected by age [17, 18], or even to be more frequent in younger individuals [8, 16].
While quantitative ODs can be assessed objectively with psychophysiological methods [19], the subjectivity of phantom smells invites to study them by letting participants fill in questionnaires about their experiences. This can even be done online, which is particularly convenient when patients cannot be approached, as was the case at the start of the COVID-19 pandemic. Usually, phantom smells are preconceived as negative by the investigators based on past experience with patients [8, 10, 14, 16]. Instead, in this article we present data obtained from an online questionnaire in which we framed questions about phantom smells in such a way that participants can describe them freely. The diversity of answers obtained with this approach called for an analysis based on Natural Language Processing (NLP), a set of techniques enabling, among other things, to reveal the valence contained in human language [20].
Given the extent of reported OD following COVID-19 (43% of COVID-19 patients worldwide according to a meta-analysis [21]) and the millions of cases over the world since the SARS-CoV-2 appeared, it is mathematical that the number of people suffering from phantosmia will increase significantly in the coming months. It is therefore important to characterize this phenomenon in order to provide the most comprehensive descriptive model possible and to better inform patients and practitioners. We further focused on how different individual characteristics could influence phantom smell perception. For instance, women are often found to react more emotionally to odors and are better at identifying odors [22]. In addition, older adults tend to report fewer emotional experiences (positive or negative) than younger adults [23]. When describing a smell, older adults also appear to use references to its characteristics more often than to its source [24]. As phantom smells are often related to something burning, smoking status may also play a role. Finally, all aspects of the associated quantitative OD (type, onset speed, persistence and duration) could impact how the phantosmia is described. With this in mind, our study has four main objectives. Firstly, we determine the prevalence of phantosmia in COVID-19 patients with OD. Secondly, we seek to identify factors modulating this prevalence, including gender, age and smoking status of the participants as well as the characteristics of their OD (type, onset speed, persistence and duration). Thirdly, we refine the study of the dynamics of the occurrence of phantosmia after contracting COVID-19 by comparing different models (linear, quadratic and logarithmic). Fourthly, we investigate the words used in the descriptions of the phantosmia, both quantitatively and qualitatively. In particular, we look at whether the number of keywords used varies between participants and at the variables that influence the valence of the descriptions or the use of certain categories of words.
Methods
Participants
Participants in an online survey (https://form.crnl.fr/index.php/146862?lang=fr) answered questions about their sociodemographic status, their COVID-19 status and their OD status (see details in [5]) between 8 April 2020 and 20 April 2021. To be included in the analysis, participants had to complete the entire questionnaire for the first time, have been positive for COVID-19 (either via a PCR test or, for participants at the beginning of the pandemic, based on their symptoms as it was a common way to detect COVID-19 due to limited access to PCR tests), and have reported having an OD. For the full inclusion criteria and inclusion tree, see Fig. S1. The final sample consisted of 4691 participants: 3763 women (80.2%) and 928 men (19.8%), with an average age of 40.4 ± 12.5 years-old (mean ± sd). Among them, 1723 were considered to have phantosmia (i.e., their descriptions fitted the definition of phantom smells).
Evaluation of phantosmia
Unlike other studies focusing on phantosmia [8, 14, 16, 18], we decided to ask an open-ended question with as little guidance as possible. In our opinion, this approach is justified by the fact that phantom smells are inherently a subjective experience. The exact formulation of our question was (originally in French): “In the last few days/weeks, have you had any olfactory hallucinations (phantom smells)?”. If the participants answered "Yes" to this question, they were then given the opportunity to freely describe these phantom smells.
Prevalence analysis
We assessed the prevalence of phantosmia in respondents with OD participating in our study (i.e., number of “yes” answers to the question about phantosmia), as well as the potential effect of seven factors on this frequency (age, gender, smoking status [smoker or non-smoker], OD type [partial/hyposmia or total/anosmia], OD onset speed [progressive or sudden], OD persistence [fluctuating or permanent] and OD duration). As the most prominent and informative effect in terms of dynamics of appearance of phantosmia was OD duration, we further explored the type of function that best fitted this relationship between phantosmia frequency and OD duration by performing a series of regression models (linear [increase or decrease?], quadratic [increase followed by decrease?], logarithmic [increase followed by plateau?]). This analysis was performed over a period of 1 to 60 weeks (i.e., 15 months) of OD duration.
Analysis of qualitative descriptions
We examined the descriptions of the phantom smells and how the seven factors cited above could modulate these descriptions.
First, we calculated the number of different keywords used by each participant to describe their phantom smell(s).
Second, in an attempt to summarize the verbal descriptions, we associated each keyword used by the participants to describe their phantom smells with one or two of the following overarching categories: “characteristic” (i.e., a characteristic of the odor, generally an adjective), “duration” (i.e., how long or how frequent the phantom smell lasts/is), “health” (i.e., health consequences of phantom smells), “location” (i.e., where the phantom smell occurs), “position” (i.e., the body position in which the phantom smell occurs) and “source” (i.e., the source of the odor). The “source” category was further divided into the following subcategories: “detergent” (i.e., toxic products), “fire” (i.e., something burning), “food” (i.e., a food item), “hydrocarbon” (i.e., fuel), “hygiene” (i.e., body hygiene), “tobacco” (i.e., tobacco use) and “others” (all remaining sources). The distribution of usage of each category shows that keywords pointing to the “source” of the phantom smell are the most frequent, followed by keywords describing a “characteristic” of the phantom smell (Fig. S2A). The 3 most common “source” subcategories are “fire”, “food” and “tobacco” (subcategory “others” aside; Fig. S2B). Therefore, we conducted analyses to determine whether these two categories (“source”, “characteristic”) and three subcategories (“fire”, “food”, “tobacco”) varied according to individual factors.
Third, we analyzed the keywords in detail by searching which keywords were more specifically used by particular groups of participants, using the tf-idf (term frequency-inverse document frequency) analysis detailed in the Supplementary Material.
Fourth, we focused on the qualitative content of the descriptions of phantosmia by following a Natural Language Processing approach. This approach allowed us to produce word clouds associated with phantosmia (Figs. S3 and S4), and to conduct a sentiment analysis of the valence (positive or negative) of the descriptions, using the R package sentimentr [25]. A sentiment analysis requires the mining of the text to be analyzed as well as an independent evaluation of the valence associated with the words used in the text, before combining these two components [26]. We first retrieved all the different keywords (N = 623 French keywords) used in the participants’ descriptions of phantom smells. Then, we conducted a new anonymous survey on a different panel of participants (N = 313 participants). This step was critical because no French lexicon based on the valence associated with odor descriptions was available. Each participant had to report its gender (women: 247 [78.9%] and men: 66 [21.1%]) and age (mean ± sd: 35.5 ± 13.7 years old) and to evaluate 30 keywords in a random order. Each keyword characterizing a phantom smell was evaluated on a valence scale ranging from − 10 (negative odor) to + 10 (positive odor). More details about the valence of the keywords can be found in the Supplementary Material.
Statistical analysis
The following analyses were performed in R.4.1.1 [27]. The statistical threshold for significance was set at α = 0.01.
The factors influencing the prevalence of phantosmia were investigated using a logistic regression with the glm() function. The presence (1) or absence (0) of phantosmia was the response variable. The explanatory variables were: (i) age, (ii) gender (men or women), (iii) smoking status (smoker or non-smoker), (iv) OD type (partial [hyposmia] or total [anosmia]), (v) OD onset speed (progressive or sudden), (vi) OD persistence (fluctuating or permanent) and (vii) OD duration. The two numeric variables (age and OD duration) were scaled before the analyses to facilitate the interpretation of the estimates. We conducted backward elimination of non-significant variables until the minimal model containing only significant variables was reached.
Then we limited our analysis to the relationship between OD duration and phantosmia prevalence in order to determine more precisely its nature. Three different models (linear, quadratic and logarithmic) were fitted to the data and their associated Akaike Information Criteria (AIC) were recorded in order to determine which model had the lowest AIC (i.e., provided a better fit to our data).
The factors influencing the number of keywords used in describing phantosmia were investigated using a generalized linear model with a positive-Poisson distribution (as all descriptions had at least one keyword) with the vglm() function from the VGAM package [28]. The same seven explanatory variables as detailed above were used and non-significant variables were dropped one by one until the minimal model was reached.
The average sentiment score associated with the descriptions of phantosmia (resulting from the previously described sentiment analysis) was investigated using a linear model with the lm() function. Again, the same seven explanatory variables were fitted in the full model and the non-significant variables were removed one by one until the minimal model was reached.
To analyze the categories used to describe phantosmia, we focused on the two main categories, “source” and “characteristic” (because they are representing 90.8% of all categories) and ran a bivariate odds ratio model (i.e., a combination of two logistic regressions in a single model) with the function vglm(). The same model selection procedure as before was followed, starting with the same seven explanatory variables.
For the three subcategories that had enough occurrence to warrant further analysis (“fire”, “food” and “tobacco”), we ran three separate logistic regressions with the same model structure and selection as before.
Results
Prevalence of phantosmia in COVID-19
Following the inclusion criteria (Fig. S1), 4691 respondents to our online questionnaire about ODs were retained and all reported OD. Among these participants, 2016 (43.0%) reported a phantosmia, while 2675 (57.0%) reported other types of OD. Based on a subjective analysis of the description of the reported phantosmia, we considered that 1723 (85.5%) truly described phantom smells (others confounded them with parosmia or their descriptions were too vague to be classified as a phantosmia). In our dataset, the prevalence of phantom smells in participants with COVID-related OD was thus 1723/4691 = 36.7%.
Factors modulating the prevalence of phantosmia
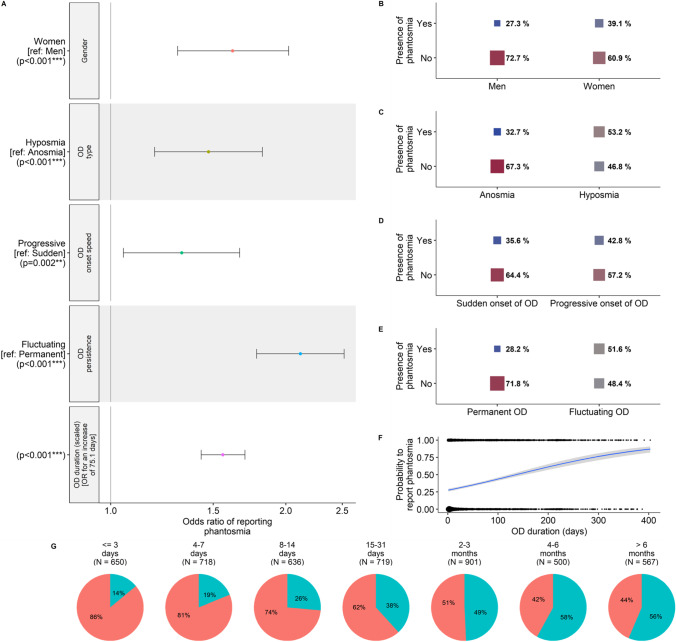
The prevalence of phantosmia was significantly affected by five of our explanatory variables (seven variables were considered: age, gender [man or woman], smoking status [smoker or non-smoker], OD type [partial/hyposmia or total/anosmia], OD onset speed [progressive or sudden], OD persistence [fluctuating or permanent] and OD duration), while two variables were non-significant (Fig. 1). Namely, the probability to report phantosmia was higher when OD was fluctuating rather than permanent (β = 0.75 ± 0.07, z = 11.2, p < 0.0001; OR [99% CI] 2.12 [1.86–2.42]), lasted longer (β = 0.44 ± 0.03, z = 13.2, p < 0.0001; OR [99% CI] 1.56 [1.46–1.67]), was partial rather than total (β = 0.39 ± 0.08, z = 4.7, p < 0.0001; OR [99% CI] 1.47 [1.25–1.73]) and appeared progressively rather than suddenly (β = 0.28 ± 0.09, z = 3.1, p < 0.01; OR [99% CI] 1.32 [1.11–1.58]). Furthermore, women were more likely to report phantosmia than men (β = 0.48 ± 0.09, z = 5.65, p < 0.0001; OR [99% CI] 1.62 [1.37–1.92]). The predicted probability to report phantosmia for a woman with a partial, fluctuating, long-lasting OD that appeared progressively was 92.9%, whereas the predicted probability to report phantosmia for a man with a total, permanent OD that appeared suddenly and did not last long was 15.4%.
Fig. 1.
Results from the logistic regression on the probability to report phantosmia (N = 4691 participants). A Odds-ratios (OR) and 99% confidence intervals of the significant variables in the minimal model. Note that for continuous variables, OR are given for each standard deviation of the corresponding variable. Effects of B gender, C OD type, D OD onset speed, E OD persistence and F OD duration on the probability to report phantosmia. G Prevalence of phantosmia (in blue) as a function of OD duration. In F, circle size is proportional to the number of participants. In B, C, D and E, square size is proportional to the percent of participants reporting phantosmia for each corresponding category, respectively
Dynamics of the appearance of phantosmia in COVID-19
When we examined the relationship between OD duration and the prevalence of phantosmia in a window of about 15 months after OD onset, results showed that the logarithmic function had a better fit to the data (AIC = 5639) than the quadratic (AIC = 5727) or the linear (AIC = 5867) function (Fig. 2). The frequency of phantosmia strongly increases during the first 8 weeks of ODs approximately, before reaching a plateau.
Fig. 2.
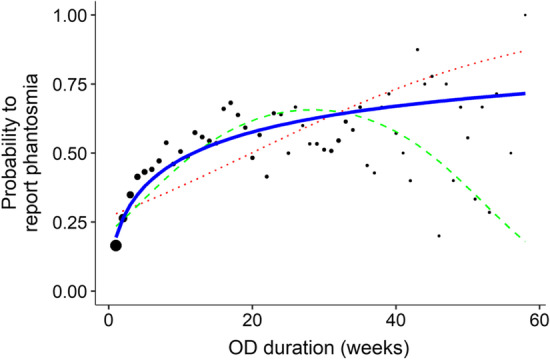
Relationship between the prevalence of phantosmia and the OD duration (N = 4691 participants). For visual clarity, OD duration has been binned per week prior to calculating the corresponding prevalence (the underlying model took into consideration the raw data). Black dots correspond to phantosmia prevalence for each week of OD duration and their size is proportional to the number of participants in this bin. The dotted red line corresponds to the linear relationship, the dashed green line corresponds to the quadratic relationship and the solid blue line [slightly bigger to underline its better fit] corresponds to the logarithmic relationship
Description of phantom smells in COVID-19
Number of descriptors
The number of keywords used to describe phantosmia was 2.43 (sd: 1.37) on average, and was not affected by any of our explanatory variables at α = 0.01: neither age, gender, smoking status nor any of the OD characteristics impacted the number of keywords used by participants to describe their phantosmia.
Keyword categories
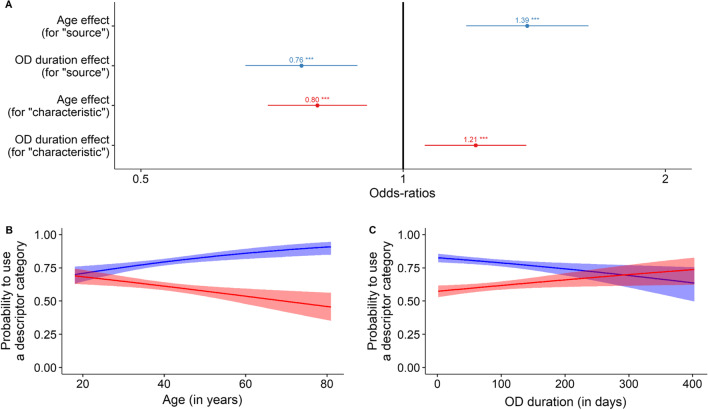
When considering which categories of words participants used to describe their phantosmia, we found that most descriptions contained a reference to the source (51.9%; e.g., “smoke”, “cigarette”) or to a characteristic of the smell (41.2%; e.g., “burnt”, “unpleasant”) (Fig. S2A). Regarding how the use of these categories vary as a function of our seven factors of interest, we found that older participants used more frequently keywords referring to the source (in blue in Fig. 3A, B; β = 0.33 ± 0.06, z = 5.2, p < 0.001; OR [99% CI]: 1.39 [1.23–1.57]) and less frequently keywords describing a characteristic (in red in Fig. 3A, B; β = −0.23 ± 0.05, z = −4.5, p < 0.001; OR [99% CI] 0.80 [0.72–0.88]). Furthermore, participants with longer OD referred more frequently to a characteristic of their phantom smell (in red in Fig. 3A, C; β = 0.19 ± 0.05, z = 3.7, p < 0.001; OR [99% CI] 1.21 [1.09–1.34]) and less frequently to its source (in blue in Fig. 3A, C; β = − 0.27 ± 0.06, z = -4.7, p < 0.001; OR [99% CI] 0.76 [0.68–0.86]).
Fig. 3.
Results from the bivariate odds ratio model (N = 1723 participants) on the probability to use a specific keyword category (either source or characteristic, excluding all other rarer categories, see “Methods”). A Odds-ratios (OR) and 99% confidence intervals of the significant variables in the minimal model. Effects of B age and C OD duration on propensity to use a keyword referring to the source (blue lines and shades) or to a characteristic (red lines and shades) of a phantom smell. Lines represent predicted probabilities of logistic regressions and shades represent their 99% confidence intervals
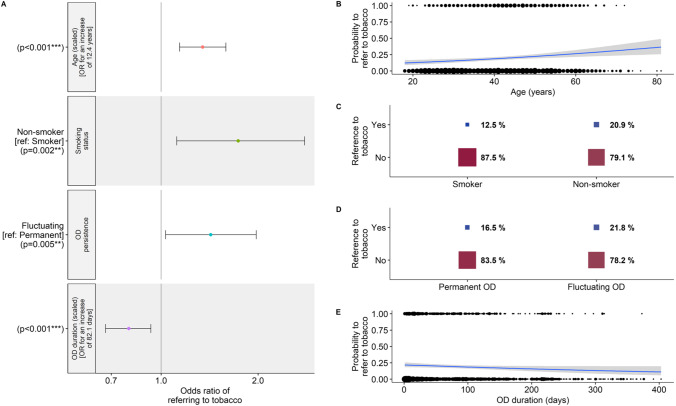
In a finer-grained analysis of the categories, we examined the most frequently cited subcategories of sources, namely “fire”, “food” and “tobacco” (Fig. S2B). None of the seven explanatory variables had an effect on the probability to make references to products or usages linked to “fire” or “food”. However, as for references to the “source” of the phantosmia, we found that older participants (β = 0.29 ± 0.06, z = 4.6, p < 0.001; OR [99% CI] 1.34 [1.14–1.58]; Fig. 4A, B) and participants with shorter OD duration (β = −0.25 ± 0.07, z = − 3.7, p < 0.001; OR [99% CI] 0.78 [0.65–0.92]; Fig. 4A, E) made more references to “tobacco” to describe their phantom smells. In addition, non-smokers made more references to “tobacco” than smokers (β = 0.55 ± 0.18, z = 3.1, p < 0.01; OR [99% CI] 1.74 [1.12–2.79]; Fig. 4A, C). Finally, participants with fluctuating OD made more references to “tobacco” than participants with permanent OD (β = 0.35 ± 0.13, z = 2.8, p < 0.01; OR [99% CI] 1.42 [1.03–1.98]; Fig. 4A, D).
Fig. 4.
Results from the logistic regression on the probability to refer to tobacco to describe phantosmia (N = 1723 participants). A Odds-ratios (OR) and 99% confidence intervals of the significant variables in the minimal model. Note that for continuous variables, OR are given for each standard deviation of the corresponding variable. Effects of B age, C smoking status, D OD persistence and E OD duration on the probability to refer to tobacco. In B, F, circle size is proportional to the number of participants. In C, D, square size is proportional to the percent of participants referring to tobacco for each corresponding category, respectively
Individual keywords
The word cloud illustrating the keywords used to describe phantosmia shows that negatively-connoted keywords are more frequent (Fig. S3), with the most frequent being “burnt” (319 occurrences, 7.63%), “smoke” (208 occurrences, 4.97%) and “cigarette” (205 occurrences, 4.90%). As age and OD duration were found to be prominent factors of variation in the previous analyses by categories, we intended to better characterize the keywords used by younger and older participants, as well as by participants with a shorter and longer OD duration. As an illustration, specific word clouds associated with young vs old and short OD vs long OD can be found in Fig. S4. The tf-idf (term frequency-inverse document frequency) analysis revealed that the 4 keywords most specific of younger participants were: “imagination”, “blood”, “fluctuating” and “stinging”. For older participants, the 4 most specific keywords were: “cigarette smoke”, “chemical”, “grilled bread” and “exhaust pipe”. For participants with shorter OD duration, the 5 most specific keywords were (the last 3 were ex-aequo): “sensation”, “blood”, “bleach”, “chlorine” and “vomit”. Finally, for participants with longer OD duration, the 4 most specific keywords were: “chemical”, “sewer”, “spicy” and “fuel”.
Sentiment analysis (valence of the descriptions)
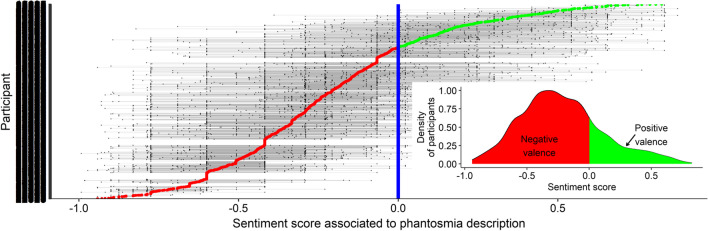
In accordance with the nature of the keywords illustrated in the word cloud (Fig. S3), the sentiments associated with the descriptions of phantosmia (derived from the evaluation of each keyword’s valence by an independent group of participants, see Methods) were negative for 77.9% of the participants and neutral or positive for the other 22.1% (Fig. 5). The average sentiment score associated with the description of phantosmia was influenced by only one of our seven explanatory variables. Participants who had a fluctuating OD described their phantosmia slightly more negatively than participants with permanent OD (β = 0.05 ± 0.02, z = 2.9, p < 0.01; Fig. S5).
Fig. 5.
Distribution of sentiments associated with the description of phantosmia (77.9% negative, 22.1% neutral or positive; N = 1723 participants). Each line represents a participant and each dot represents a keyword used by the participant to describe his/her phantosmia (keywords used by the same participant are connected by thin grey lines). The bigger red (negative descriptions) and green (positive descriptions) dots represent the average sentiment score of each participant (participants are sorted based on this average score). The vertical blue line represents a neutral evaluation, while scores between − 1.0 and 0.0 represent negative descriptions and scores between 0.0 and + 1.0 represent positive descriptions. The sentiment value associated with each keyword was determined by an independent sample of participants on a scale from − 10 to + 10 (see “Methods”). The inset on the bottom right represents the density distribution of the average sentiment score of the participants (negative descriptions in red and positive descriptions in green)
Discussion
COVID-19 has affected many people over the world, often with associated OD. This pandemic therefore represents a unique occasion to study a previously rare, but unfortunately increasing, qualitative OD: phantosmia. By analyzing the responses of more than 4500 individuals, we showed that the prevalence of phantosmia in COVID-19 patients with OD was very high (37% of 4691 people). This prevalence was influenced by the gender of the participants (more frequent in women), and the probability to report phantosmia differed as a function of OD characteristics (higher in fluctuating, long-lasting, partial ODs that progressively settled in). In particular, the prevalence of phantosmia ranged from 14% at the very beginning of the olfactory symptoms of COVID-19 to 56% after 6 months. The dynamics of the prevalence of phantosmia as a function of OD duration is best explained by a logarithmic relationship, with a strong increase at the beginning subsequently followed by a plateau.
The prevalence of phantosmia found in our study was in line with other studies using online questionnaires completed by participants of different countries: up to 34% of COVID-19 patients with OD [11] and 31% of patients with more varied etiologies of OD [29], for instance. Such a high percentage may result from a selection bias in our online questionnaire (among other limits of the approach, which are detailed in [5]). People who are the most affected by their ODs, including persons with phantosmia, may have been more likely to spontaneously participate to our study. However, this high prevalence may also stem from a known association of phantosmia with depression [30], the global prevalence of which has drastically increased during the pandemic [31]. The fact that women appeared to be more prone to phantom smells than men confirms some previous findings [8, 16], but not all [10]. Age did not seem to be an influential factor in our study, while higher prevalence of phantosmia have been reported elsewhere in younger participants [8, 16].
Of particular interest for the understanding of this olfactory phenomenon are the relationship between its occurrence and OD characteristics. First, evidence is pointing towards the necessity of having an at least partially functioning olfactory system to experience phantom smells. Indeed, we found that phantom smells were more frequently reported when OD appeared progressively than when it appeared suddenly. Phantom smells were more prevalent in hyposmic than in anosmic patients, and in fluctuating (vs. permanent) ODs. Second, as also showed in another group of COVID-19 participants [11], we found that the prevalence of phantosmia increases as the duration of the smell disorders associated with COVID-19 increases. While there were anecdotal reports of very brief episodes of phantosmia on the day preceding the total loss of smell in a few patients, in most cases phantosmia occurs in a delayed fashion, sometimes even after apparent recovery as this starts to be reported in case studies for other qualitative disorders [12]. This is consistent with Leopold [6]’s statement that olfactory distortions (including phantosmia) seem to occur either during olfactory receptor neuron death or regeneration. It is noteworthy that COVID-19 patients experiencing phantosmia often have an ability to smell (quantitatively) within the normal range (6 patients out of 9 with phantosmia were normosmic while the others were hyposmic in [9] and patients with phantosmia and/or parosmia did not differ in Sniffin’ Sticks test scores from patients without qualitative ODs in [32]).
Although studies are clearly needed to better characterize the pathophysiology of phantosmia, several hypotheses about peripheral and central mechanisms (which are not necessarily exclusive) of such a phenomenon have been formulated. At the peripheral level, lower number of olfactory neurons in the olfactory epithelium, higher number of immature neurons and disordered growth of olfactory axons have been found in patients with phantosmia [6]. Peripheral phantosmia is more often intermittent and worse on one side, relieved by nasal obstruction and anesthesia/resection of the olfactory epithelium [33]. Some reports in our study indicated that mechanical actions affecting the nasal cavity, such as yawning or blowing one’s nose, could trigger a phantom smell [5]. Central mechanisms may also occur, with manifestations that are constant, bilateral and not relieved by any of the options mentioned previously [33]. This is consistent with abnormally high brain activity in several frontal, insular and temporal regions [6], but also with some etiologies of phantosmia outside COVID-19 (psychiatric diseases, neurologic and neurodegenerative disorders). Consistent with the central hypothesis, the reports of several patients in our study were in favor of an effect of suggestion (like reading/talking about a smell, which would trigger the phantom smell) and of attention (phantosmia being more present during the peak periods of the epidemic waves, or disappearing during a limited period in which the patient has changed environment and directed her attention to a person to help). Representation of an odor can be elicited in people without pathological condition (imagined odor: [34]). Possible dysfunction or damage in the central olfactory pathways (olfactory bulb, olfactory tract and/or primary/secondary cortices) [33] could trigger such representations. It has been suggested that disinhibition of olfactory excitation could originate in these unwanted odor perceptions.
The shape of the prevalence curve (Fig. 2) suggests that there might be both peripheral and central phenomena in play. The prevalence of phantosmia reaches a plateau at a time (about 8 weeks) were neuronal regeneration in the olfactory epithelium is likely to take place: indeed, regeneration time of a healthy epithelium after axotomy (including olfactory bulb reinnervation) is about 30 days in mammals [35, 36], but could be longer in a damaged epithelium. From this time on, the prevalence curve then illustrates what seems to be a rather installed phenomenon, since it does not decrease over 60 weeks after the beginning of the first COVID-19-related OD. This is particularly preoccupying first because, whereas parosmia seems to be a positive sign of recovery, phantosmia appears to be a poorer predictor of recovery in the most recent studies in COVID-19 patients [37] as well as in patients with varied etiologies [38] (but see [39] for contradictory findings that occurrence of parosmia or phantosmia has little prognostic value). And second, because to date there is not enough evidence in the literature to formulate treatment recommendations for phantosmia (or parosmia): only anecdotal evidence can be found for the local use of some medical therapies, such as antimigraines, antipsychotics or antiepileptic, with success rates depending on the patients’ etiology [33] (see also the recent study by [40] for an encouraging effect of intranasal sodium citrate in reducing phantosmia).
Additionally, with regards to the qualitative description of phantom smells, we found that 78% of the participants described their phantosmia as a negative experience, and this was more marked when the OD was fluctuating. Why phantom smells are more often unpleasant is an intriguing question, to which we can propose several possible answers. First, one of the main functions of olfaction, which has the most immediate consequences for survival, is the detection of threats. One can thus hypothesize that, (i) in the case of anarchic activation of olfactory neurons and (ii) assuming there is a central contribution to the generated olfactory percept, olfactory representations that are preferentially generated are those of odors that are the most relevant for survival (smoke, decay/fermentation…). The fact that the odor of a toxic substance, tobacco, was cited more often by non-smokers as a phantom smell is totally in line with this, given that the threatening value of tobacco products is likely to be stronger in this subgroup. Second, the dimensions of unexpectedness (phantom smells occurring in a non-predictable manner) and incongruency (phantom smells unrelated with the actual physical environment) may contribute significantly to the unpleasantness of this experience since they are significant determinants of the responses to smells [41]. Finally, fluctuation of the occurrence of phantom smells is likely to worsen the deleterious effect of unexpectedness, explaining why fluctuating OD is a significant predictor of negativity of phantosmia.
Another result of interest is that participants with OD of shorter duration and older participants tended to favor source names (i.e., descriptions of the olfactory experience). Conversely, participants with OD of longer duration and younger participants referred more to odor characteristics (i.e., more emotional descriptions of the olfactory experience). The fact that older participants used more words linked to the potential source of the phantom smell than to its characteristics contrasts with previous results [24], which found the opposite pattern when participants were asked to describe an actually perceived odorant. It could therefore be that semantic usage differs with age depending on whether participants have to describe a real or a mental construction of a smell. In addition, as elderly persons are typically less sensitive to emotions [23], it could be that older participants use less emotional descriptors and thus refer more to the source of the smell. The different usage of semantic categories with age may be linked to age-related changes in word representation and retrieval [42]. Regarding the effect of OD duration, it is possible that people with longer OD are more annoyed by the phantom phenomenon and use more adjectives to describe how they feel about it whereas people with shorter OD are still in the exploratory phase where they have a more analytical approach, trying to define what the odor is exactly. More broadly, it is worth mentioning that phantosmia is subjective and may be affected by the usage of a specific language. Future studies could therefore try to assess how phantom smells are described by respondents from different cultures and/or languages with different sizes of smell-related vocabularies [43].
Finally, we would like to stress the importance of studying phantosmia separately from parosmia. These qualitative ODs are often grouped together in the literature, but it has been suggested that this may be a mistake since they have different patterns of expression depending on demographic factors, etiologies and consequences on the quality of life [10]. Although adopting a questionnaire approach has limitations [5], it provides useful quantitative and qualitative elements to gain additional insights into previously rarely observed phenomena such as phantosmia. Future studies are needed to better understand this category of sensory hallucinations and its physiopathology. As well as parosmia, phantosmia has very deleterious consequences on the patients’ quality of life [44]. In spite of this, knowledge of these and other related ODs remains low amongst medical professionals [5, 45] and the medical community is still lacking therapeutic options [29]. Hopefully, the number of studies on phantosmia, which already significantly increased in 2020 and 2021, will continue to grow in the future to better answer the needs of the many patients suffering from this long-term sequalae of COVID-19.
Conclusion on clinical relevance
By using a model of viral infection often associated with olfactory disorders, COVID-19, we pointed at the high frequency of the under-studied phenomenon of phantom smells in patients with post-infectious ODs. Indeed, using spontaneous reports of patients with an online questionnaire, we found that 37% of post-COVID patients with ODs experienced phantom smells. It is important to note that this figure is likely to overestimate the prevalence of this symptom on the ground, since people who suffer from their OD may be over-represented within the sample of volunteers who answered the online questionnaire. It may though be in line with the frequency observed by the clinicians because patients who decide to consult for their phantosmia are those who are adversely affected by their condition. The characteristics of phantom smells (i.e., which smells, (un)pleasantness of the smells) and their dynamics of occurrence after OD onset, as we report them in this article, are certainly well representative of the reality on the ground. To better inform patients, clinicians’ attention should be drawn to the factors associated with a higher probability to develop phantosmia, namely being a woman and displaying a fluctuating/long-lasting/progressively installed OD. Finally, it must be kept in mind by the medical and scientific community that research on the characteristics, mechanisms and remediation of phantosmia is dependent on patients’ verbal reports, since there is no objective way to measure sensory hallucinations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank all the people who helped disseminate the questionnaire. This work was carried out with the financial support of the IDEXLYON Project of the University of Lyon as part of the Future Investments Program (ANR-16-IDEX-0005, CORODORAT project to CF and MB).
Author contributions
Conceived and designed the study: MB, CF. Wrote the paper: CB, MB, CF. Data acquisition and curation: CB, KB, MB, CF. Performed analysis: CB, KB. Edited and approved the final manuscript: CB, KB, MB, CF.
Declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christophe Bousquet and Kamar Bouchoucha: co-first authors.
Moustafa Bensafi and Camille Ferdenzi: co-last authors.
Contributor Information
Moustafa Bensafi, Email: moustafa.bensafi@cnrs.fr.
Camille Ferdenzi, Email: camille.ferdenzi@cnrs.fr.
References
- 1.Hoang MP, Kanjanaumporn J, Aeumjaturapat S, et al. Olfactory and gustatory dysfunctions in COVID-19 patients: a systematic review and meta-analysis. Asian Pac J Allergy Immunol. 2020;38:162–169. doi: 10.12932/AP-210520-0853. [DOI] [PubMed] [Google Scholar]
- 2.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boscolo-Rizzo P, Menegaldo A, Fabbris C, et al. Six-month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem Senses. 2021 doi: 10.1093/chemse/bjab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdenzi C, Bousquet C, Aguera P-E, et al. Recovery from COVID-19-related olfactory disorders and quality of life: insights from an observational online study. Chem Senses. 2021;46:1–10. doi: 10.1093/chemse/bjab028. [DOI] [PubMed] [Google Scholar]
- 6.Leopold D. Distortion of olfactory perception: diagnosis and treatment. Chem Senses. 2002;27:611–615. doi: 10.1093/chemse/27.7.611. [DOI] [PubMed] [Google Scholar]
- 7.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Oto-Rhino-Laryngol Head Neck. 2005;262:231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 8.Bainbridge KE, Byrd-Clark D, Leopold D. Factors associated with phantom odor perception among US adults: findings from the National Health and Nutrition Examination Survey. JAMA Otolaryngol Neck Surg. 2018;144:807–814. doi: 10.1001/jamaoto.2018.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.İşlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine case. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegrino R, Mainland JD, Kelly CE, et al. Prevalence and correlates of parosmia and phantosmia among smell disorders. Chem Senses. 2021;46:bjab046. doi: 10.1093/chemse/bjab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohla K, Veldhuizen MG, Green T, et al. Increasing incidence of parosmia and phantosmia in patients recovering from COVID-19 smell loss. medRxiv. 2021 doi: 10.1101/2021.08.28.21262763. [DOI] [Google Scholar]
- 12.Duyan M, Ozturan IU, Altas M. Delayed parosmia following SARS-CoV-2 infection: a rare late complication of COVID-19. SN Compr Clin Med. 2021;3:1200–1202. doi: 10.1007/s42399-021-00876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannier S, Berdagué JL, Rieu I, et al. Prevalence and phenomenology of olfactory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:1019–1021. doi: 10.1136/jnnp-2012-302414. [DOI] [PubMed] [Google Scholar]
- 14.Nordin S, Murphy C, Davidson TM, et al. Prevalence and assessment of qualitative olfactory dysfunction in different age groups. Laryngoscope. 1996;106:739–744. doi: 10.1097/00005537-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Bussiere N, Mei J, Levesque-Boissonneault C, et al. Chemosensory dysfunctions induced by COVID-19 can persist up to 7 months: a study of over 700 healthcare workers. Chem Senses. 2021;46:bjab038. doi: 10.1093/chemse/bjab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sjölund S, Larsson M, Olofsson JK, et al. Phantom smells: prevalence and correlates in a population-based sample of older adults. Chem Senses. 2017;42:309–318. doi: 10.1093/chemse/bjx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laloyaux J, Badcock JC. Effects of age on hallucinations in the general population: a commentary on recent data and challenges for future research. Psychiatry Res. 2020;291:113253–113253. doi: 10.1016/j.psychres.2020.113253. [DOI] [PubMed] [Google Scholar]
- 18.Larøi F, Bless JJ, Laloyaux J, et al. An epidemiological study on the prevalence of hallucinations in a general-population sample: effects of age and sensory modality. Psychiatry Res. 2019;272:707–714. doi: 10.1016/j.psychres.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Manesse C, Ferdenzi C, Mantel M, et al. The prevalence of olfactory deficits and their effects on eating behavior from childhood to old age: a large-scale study in the French population. Food Qual Prefer. 2021;93:104273. doi: 10.1016/j.foodqual.2021.104273. [DOI] [Google Scholar]
- 20.Dodds PS, Clark EM, Desu S, et al. Human language reveals a universal positivity bias. Proc Natl Acad Sci USA. 2015;112:2389–2394. doi: 10.1073/pnas.1411678112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferdenzi C, Roberts SC, Schirmer A, et al. Variability of affective responses to odors: culture, gender, and olfactory knowledge. Chem Senses. 2013;38:175–186. doi: 10.1093/chemse/bjs083. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Ballesteros R, Fernández V, Cobo L, et al. Do inferences about age differences in emotional experience depend on the parameters analyzed? J Happiness Stud. 2010;11:517–521. doi: 10.1007/s10902-009-9169-y. [DOI] [Google Scholar]
- 24.Poncelet J, Rinck F, Ziessel A, et al. Semantic knowledge influences prewired hedonic responses to odors. PLoS ONE. 2010;5:e13878. doi: 10.1371/journal.pone.0013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinker TW (2019) sentimentr: calculate text polarity sentiment
- 26.Bhadane C, Dalal H, Doshi H. Sentiment analysis: measuring opinions. Procedia Comput Sci. 2015;45:808–814. doi: 10.1016/j.procs.2015.03.159. [DOI] [Google Scholar]
- 27.R core team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
- 28.Yee TW. The VGAM package. R News. 2008;8:28–39. [Google Scholar]
- 29.Philpott C, Dixon J, Boak D. Qualitative olfactory disorders: patient experiences and self-management. Allergy Rhinol. 2021;12:21526567211004252. doi: 10.1177/21526567211004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croy I, Yarina S, Hummel T. Enhanced parosmia and phantosmia in patients with severe depression. Psychol Med. 2013;43:2460–2464. doi: 10.1017/S0033291713001773. [DOI] [PubMed] [Google Scholar]
- 31.Santomauro DF, Mantilla Herrera AM, Shadid J, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ercoli T, Masala C, Pinna I, et al. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol Sci. 2021;42:4921–4926. doi: 10.1007/s10072-021-05611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saltagi MZ, Rabbani CC, Ting JY, Higgins TS. Management of long-lasting phantosmia: a systematic review. Int Forum Allergy Rhinol. 2018;8:790–796. doi: 10.1002/alr.22108. [DOI] [PubMed] [Google Scholar]
- 34.Bensafi M, Porter J, Pouliot S, et al. Olfactomotor activity during imagery mimics that during perception. Nat Neurosci. 2003;6:1142–1144. doi: 10.1038/nn1145. [DOI] [PubMed] [Google Scholar]
- 35.Monti Graziadei GA, Graziadei PPC. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- 36.Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of thefila olfactoria in rat. J Neurocytol. 1980;9:145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- 37.Teaima AA, Salem OM, Teama MAEM, et al. Patterns and clinical outcomes of olfactory and gustatory disorders in six months: prospective study of 1031 COVID-19 patients. Am J Otolaryngol. 2022;43:103259. doi: 10.1016/j.amjoto.2021.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu DT, Sabha M, Damm M, et al. Parosmia is associated with relevant olfactory recovery after olfactory training. Laryngoscope. 2021;131:618–623. doi: 10.1002/lary.29277. [DOI] [PubMed] [Google Scholar]
- 39.Reden J, Maroldt H, Fritz A, et al. A study on the prognostic significance of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 2007;264:139–144. doi: 10.1007/s00405-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 40.Whitcroft KL, Gunder N, Cuevas M, et al. Intranasal sodium citrate in quantitative and qualitative olfactory dysfunction: results from a prospective, controlled trial of prolonged use in 60 patients. Eur Arch Oto-Rhino-Laryngol. 2021 doi: 10.1007/s00405-020-06567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manesse C, Fournel A, Bensafi M, Ferdenzi C. Visual priming influences olfactomotor response and perceptual experience of smells. Chem Senses. 2020;45:211–218. doi: 10.1093/chemse/bjaa008. [DOI] [PubMed] [Google Scholar]
- 42.Wulff DU, Deyne SD, Jones MN, et al. New perspectives on the aging lexicon. Trends Cogn Sci. 2019;23:686–698. doi: 10.1016/j.tics.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Majid A. Human olfaction at the intersection of language, culture, and biology. Trends Cogn Sci. 2021;25:111–123. doi: 10.1016/j.tics.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Landis BN, Reden J, Haehner A. Idiopathic phantosmia: outcome and clinical significance. ORL J Oto-Rhino-Laryngol Its Relat Spec. 2010;72:252–255. doi: 10.1159/000317024. [DOI] [PubMed] [Google Scholar]
- 45.Ferdenzi C, Bellil D, Boudrahem S, et al. La rééducation olfactive: bénéfices d’une prise en soins pluri-professionnelle. Presse Médicale Form. 2021 doi: 10.1016/j.lpmfor.2021.11.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.