Abstract
Introduction
Vaccines are being developed against Group B Streptococcus and respiratory syncytial virus. These vaccines are designed to be given to pregnant women to protect infants; thus, their success depends on uptake in this population. Maternal immunization programs have struggled to achieve target coverage rates. This systematic narrative synthesis aims to define the most important barriers and facilitators for maternal immunization and to identify priority areas for future research.
Methods
A search strategy was developed in Medline and adapted according to the requirements of additional search engines. Two reviewers independently reviewed the studies, using pre-specified inclusion and exclusion criteria. Results sections of included studies were coded, and thematic analysis was used to identify prominent themes.
Results
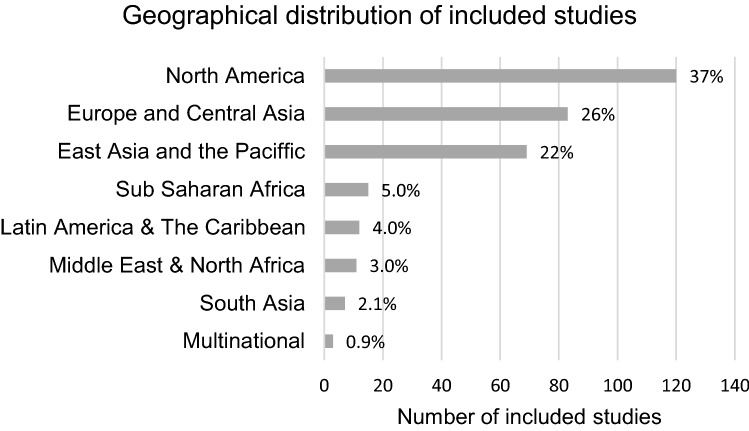
321 studies were included in the final review. Most studies came from North America (37%), Europe (26%) or East Asia, Australia and New Zealand (22%). Low-and middle-income countries were under-represented. Five percent of studies came from Sub-Saharan Africa, and 2% came from South Asia. The prominent factors impacting maternal immunization were provider recommendation, perceived risks and benefits of maternal vaccines for the infant, race, birthplace, and access to healthcare. Few studies explored reasons behind racial and socioeconomic disparities in maternal immunization rates.
Discussion
A strong provider recommendation, equitable access to prenatal care and messaging that focuses on vaccine safety and infant benefits emerged as the key components for optimising vaccine uptake among pregnant women. Research among healthcare providers, minority groups and in low- and-middle-income countries was lacking. In anticipation of the expansion of maternal immunization programmes, focused research is needed to address these gaps and inform a successful public health strategy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10995-022-03508-0.
Keywords: Vaccine, Pregnancy, Hesitancy, Global, Maternal immunization
Significance
What is already known on this topic? Maternal immunization is an expanding platform for protecting both women and vulnerable infants from infection. Vaccines that target Group B Streptococcus and respiratory syncytial virus are in clinical trials. Additionally, COVID-19 vaccination is recommended for all pregnant women. Existing maternal immunization programmes across the world struggle to achieve adequate vaccination coverage.
What does this review add? This review synthesises 10 years of research on this topic and gives a global perspective on the factors impacting uptake of vaccines in pregnant women. The review identifies the key drivers for uptake of vaccines in pregnancy. The results reveal important racial and ethnic disparities in maternal immunization rates across many different geographic regions and highlight the need for in depth, qualitative research to understand the reasons for such disparities in different geographic settings.
Introduction
Vaccines have significantly contributed to reduction in child mortality. The measles vaccine alone is estimated to have saved 25.5 million lives since the year 2000 (Patel et al., 2020). However, progress has been slower in reducing deaths in infants that are too young to receive vaccines. Vaccines currently licensed and recommended during pregnancy protect pregnant women and their new-born infants from influenza, pertussis, and tetanus. Vaccination during pregnancy can have different primary goals. The first is to protect the pregnant woman from severe infection and death, the second is to protect the infant through the transfer of maternal antibodies. Pregnant women are vaccinated against influenza and SARS-CoV-2 [causative agent of coronavirus disease 2019 (COVID-19)] because they are at increased risk of severe infection and death from these infections. On the other hand, pertussis vaccines are given to protect the infant from severe consequences of pertussis infection (Beigi et al., 2014; Moniz & Beigi, 2014; Omer, 2017). Vaccines that target Group B streptococcus (GBS) and respiratory syncytial virus (RSV) are in development. These vaccines use a maternal immunization platform to prevent GBS and RSV in young infants (Heath et al., 2017). COVID-19 vaccines are recommended for all pregnant women. The success of any new vaccine will depend on achieving good uptake in the population. To date, achieving optimal levels of maternal vaccination has been a global challenge (Omer, 2017). Population based data on maternal immunization uptake is limited. In countries such as the United States (US), where there is available population-based data, there has been a steady increase in uptake of pertussis and influenza vaccine during pregnancy. Rates, however, remain suboptimal. In the US in the 2019–2020 season, 61.2% of pregnant women received influenza vaccination and 56.6% received Tdap during pregnancy (Razzaghi et al., 2020). The COVID-19 pandemic has again highlighted challenges with acceptance of vaccines during pregnancy. The objectives of this review are to synthesise the current global literature on barriers and facilitators to vaccination during pregnancy and to identify knowledge gaps to inform the progress of impactful research in this field.
Methods
Search Strategy
This review was guided by the PRISMA check list for reporting systematic reviews. A search strategy was developed in Medline and adapted according to the specific indexing requirements of additional data sources. The search was carried out in Medline, Excerpta Medica database (EMBASE®), Cumulative Index to Nursing and Allied Health Literature (CINAHL), International Bibliography of the Social Sciences (IBSS), PsycINFO and Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS). The search took place on 02/13/2019 (Table 1). A repeat Medline search on 1/19/2021 updated the review.
Table 1.
Search terms and limitation criteria
| Key words used in Search |
| Physician practice patterns [Mesh] OR attitude of health personnel [Mesh] OR anxiety OR fear OR doubt OR Vaccination Coverage [Mesh] OR patient acceptance of health care [Mesh] OR uptake OR refusal OR acceptance OR health knowledge, attitudes, practice [Mesh] OR attitude* OR knowledge OR barrier* OR hesitancy OR practice |
| AND |
| Pregnancy OR pregnant OR “Pregnant Women” [Mesh] OR childbearing OR maternal OR post- partum OR puerperium |
| AND |
| Vaccine OR “Vaccines” [Mesh] OR “Immunization” [Mesh] OR vaccines OR vaccination OR immunizations OR vaccinated OR immunization OR immunized OR vaccinate |
| Additional limits |
| Publication date: 01/01/2010–02/13/2019. Updated search: 02/14/2019–01/19/2021** |
| Data Sources used: Medline, EMBASE, CINAHL, Psych info, IBSS, *Key words developed in Medline and adapted according to indexing requirements for the other data sources. **Updated search conducted in Medline |
Study Selection
After removing duplicates, studies were screened for inclusion by title and abstract, then by full text review according to pre-defined criteria. All English language publications of primary research examining barriers and facilitators to vaccination during pregnancy were included. Review articles and opinion pieces were excluded. Vaccine coverage studies were included only if they also examined factors associated with vaccine uptake. Full details of inclusion and exclusion criteria are provided in Table 2. Two authors, SG and SS independently reviewed the studies. Discrepancies were discussed among all the authors and resolved through consensus agreement.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Primary published research that examines facilitators and barriers to maternal immunization Studies examining both currently recommended vaccines (Tdap, TT, TD, Influenza) and potential future vaccines (ZIKA, RSV, GBS, CMV) should be included |
Review articles & opinion pieces Vaccine safety, efficacy, and cost effectiveness studies Implementation studies unless there is a relevant baseline study Reports of vaccine coverage alone that do not include any population demographics Publication not in English |
Data Extraction
Data was extracted using thematic coding of the results section of each study. A codebook was developed based on the SAGE working group determinants of vaccine hesitancy (MacDonald, 2015). Software (NVivo version 12) was used to attach the codes to the results sections. Two authors SG and SS independently coded the same 50 articles and met weekly to adapt the codebook as needed. Once coders established agreement and no new codes were being added, the remainder of the articles were coded by one author according to the final codebook. Reviewers continued to meet weekly throughout the coding process. A full codebook with definitions is included as supplemental data.
Appraisal of Included Studies
We used the Centre for evidence-based management (CEBM) tool for critical appraisal of a cross sectional study to evaluate quantitative survey-based studies (CEBM, 2014). This tool is a 10-point check list that focuses on the risk of sampling bias and validity of survey instruments. The Critical Appraisal Skills Program (CASP) qualitative research checklist was used to assess qualitative and mixed methods research. This 10-point check list evaluates rigour and examines the relevance of the results (Noyes et al., 2018). Two reviewers (SG and SS) independently reviewed the quality of the studies. All studies were included regardless of quality to ensure that important themes were not excluded (Noyes et al., 2018).
Ethics committee approval was not required as this manuscript is a review article. The manuscript is not based on patient data or a clinical study.
Results
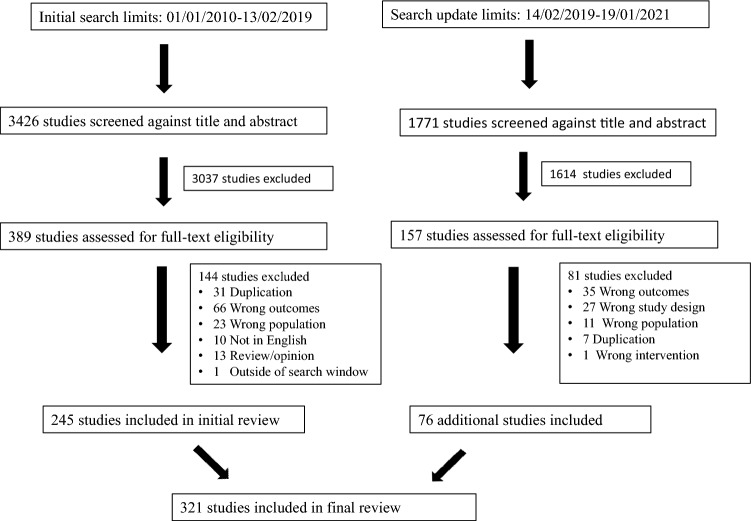
The initial searches identified 5197 studies. After screening by title and abstract 546 studies remained for full text review. A further 225 studies were excluded following full text review. The final review included 321 studies. A PRISMA diagram describing study selection is shown in Fig. 1. A full list of the selected studies is included as supplemental data.
Fig. 1.
PRISMA flow diagram for study selection showing both searches. The initial searchwhich took place on 02/13/2019 and a repeat Medline search on 1/19/2021
Characteristics of Included Studies
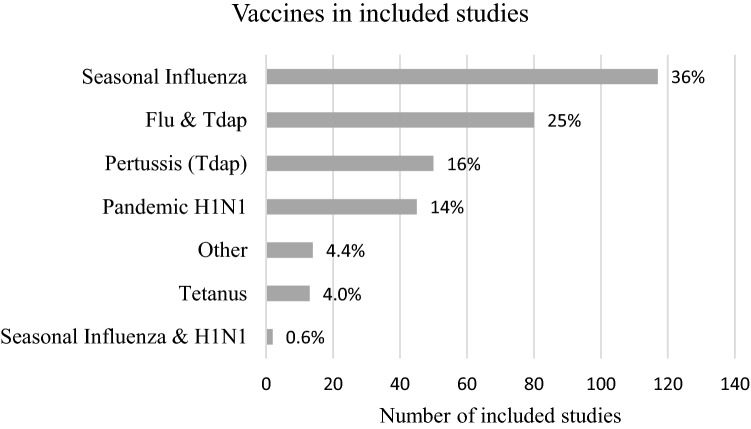
Most of the studies came from North America (37%), Europe (26%) or East Asia, Australia and New Zealand (22%) (Fig. 2). Influenza vaccine and/or Tetanus diphtheria and acellular pertussis (Tdap) vaccine were the most common vaccines evaluated in included studies (77%) (Fig. 3). Eighty-four percent of studies used quantitative methods and 16% used qualitative or mixed methods. Pregnant or childbearing women were the subjects in 77% of studies, 14% focused on prenatal care providers alone and 7% included both providers and pregnant women.
Fig. 2.
Geographic distribution of included studies
Fig. 3.
Vaccines mentioned in included studies
Quality Assessment
Among the 269 quantitative studies, participant selection was clearly described in 80% of studies. There was a risk of selection bias identified in 50% of studies and 30% used a reliable and validated survey instrument. Among the qualitative and mixed methods research, 85% were deemed to be moderately or highly valuable and 57% had appropriate recruitment, data collection and rigorous analysis. Quality assessment of each of the included studies is available on request.
Main Findings
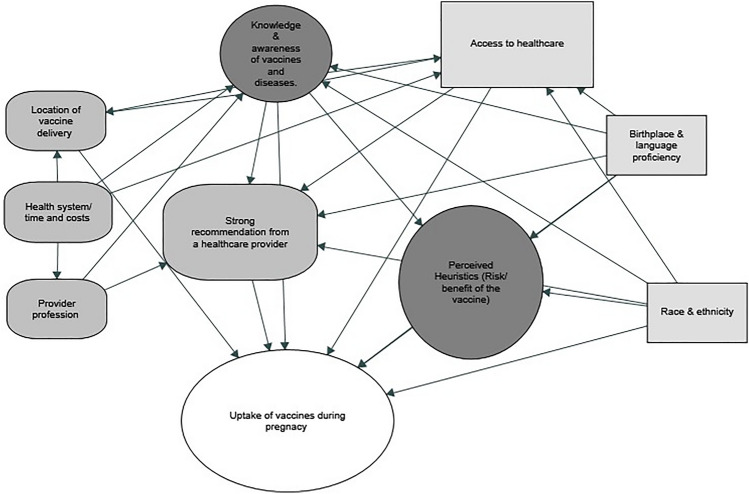
Across 321 studies, a broad range of factors impacting maternal immunization was described. These factors were grouped into ‘contextual’, ‘social and individual group influences’ and ‘vaccine specific’ factors as defined in the SAGE working group determinants of vaccine hesitancy (MacDonald, 2015). Among these factors, social and individual group influences were the most frequently identified factors, particularly perception of risks and benefit of vaccines (perceived heuristics) and provider recommendation. Among contextual factors, socioeconomic group factors including access to healthcare, race, ethnicity, and birthplace were pervasive themes across multiple geographic regions. Among vaccine and vaccine specific uses, the roles of health care professionals and location of immunization services were the most important themes (Fig. 4).
Fig. 4.
Interplay of factors influencing uptake of vaccines in pregnancy, grouped according to the SAGE working group’s determinants of vaccine hesitancy.  Contextual (sociodemographic/geo-political),
Contextual (sociodemographic/geo-political), 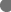 Individual and social group influences,
Individual and social group influences,  Vaccine specific uses
Vaccine specific uses
Individual and Social Group Influences
The dominant theme to emerge from the included studies was individual perception of the risks and benefits of recommended vaccines (Fig. 4). Among perceived heuristics, the most important barrier was concern about safety. A recent meta-analysis found that the odds of accepting influenza vaccine during pregnancy were 86% lower when women perceived the vaccine to be unsafe (OR 0.22, 95% CI 0.11–0.44, seven studies) (Kilich et al., 2020). The strongest motivator for pregnant women is desire to protect their infant from infection. There were 23 qualitative studies with pregnant women included in this review. Protection of the foetus and infant was a key theme to emerge from these studies, both as a motivating factor and the primary safety concern.
Among prenatal providers concerns about safety and perceptions of disease severity and susceptibility were identified as important motivators and or barriers to recommending vaccines to pregnant women. In a survey of 1061 maternity care providers in Canada the most common reasons for not recommending the flu vaccine were that potential risks were not outweighed by benefits, suboptimal efficacy, concerns about safety and lack of information about recommended vaccines (Dubé et al., 2018). Education of providers was also important, in a study of 3304 midwives, practice nurses and health visitors in the United Kingdom (UK), 56% stated that they had not received any training on vaccines for pregnant women (Vishram et al., 2018). Similar findings were reported in a survey of Australian midwives where the majority of participants felt they had insufficient training and those midwives who perceived their training to be adequate were more likely to report feeling confident in vaccine communication with patients (Frawley et al., 2020). In a study of 476 Korean obstetricians, lack of knowledge on vaccine safety and severity of influenza was identified as an important barrier (Noh et al., 2016).
Contextual Influences
Access to Healthcare Adequate prenatal care was frequently cited as a factor associated with maternal vaccination, particularly in low-and middle-income settings. In a well-designed community based cross-sectional study of women of childbearing age in Dukem, Ethiopia, both distance from a health care facility and attending follow up antenatal care visits were associated with tetanus toxoid vaccine uptake on adjusted analysis (Anatea et al., 2018). Research in Pakistan, Ivory coast, Sierra Leone, Peru and Brazil demonstrated an association between increased vaccine uptake and increased number of prenatal care visits (Arriola et al., 2021; Iqbal et al., 2020; Mendoza-Sassi et al., 2015, 2019; Yaya et al., 2019, 2020). In a mixed-methods study examining barriers and facilitators to implementing a national maternal influenza vaccine strategy in El Salvador, barriers to accessing care included unwillingness of employers to allow time off work for prenatal visits and gang violence, highlighting that reasons for lack of access to prenatal care can be broad and context specific (Fleming et al., 2018).
Lack of access to adequate prenatal care is also a problem in high-income countries. In a study of 113,730 live births in Minnesota, inadequate prenatal care was associated with decreased Tdap uptake in pregnant women (Barber et al., 2017). Similar findings were reported in a study of 3132 pregnant women attending a large public hospital in Atlanta, Georgia (Doraivelu et al., 2019). In a nationally representative sample of 13,543 women who gave birth in France, the H1N1 vaccination rate was much lower in women with inadequate prenatal care however inadequate care was not found to be independently associated with vaccine uptake when adjusted for other socio-demographic factors (Blondel et al., 2012).
Twelve studies in the United Stated (US) found that influenza and Tdap vaccine uptake during pregnancy was lower in women with government funded insurance (Medicaid) compared with women with private insurance (Koerner et al., 2018; Merritt et al., 2020; New et al., 2018; Wales et al., 2020). Insurance status was also found to impact uptake in studies from Europe and Australia (Crosby et al., 2016; Regan et al., 2016).
Race and Ethnicity Race and ethnicity emerged as a factor impacting vaccine uptake in studies in North America, Australia, Europe, and Asia. This theme was most frequently examined in studies from the US. The US Pregnancy Risk Assessment Monitoring System (PRAMS) has provided state-based estimates of influenza vaccination during pregnancy since 2009 (Kennedy et al., 2012). Three national reports, and two state-based reports using PRAMS data ranging from 2009 to 2017 were included in this review. All reports found that uptake in Non-Hispanic Black women was lower than uptake in white or Hispanic women (Ding et al., 2014, 2015, 2017; Howland, 2013). In a secondary analysis of 3 years of PRAMS data (2012–2015) which included survey responses from 130,161 pregnant women, Non-Hispanic Black women were 30% less likely to receive influenza vaccine during pregnancy, controlling for maternal age, marital status, education, and prenatal care utilization (Arnold et al., 2019).
We found few studies examining the reasons for these disparities. One well-designed qualitative study examined messaging strategies that might increase influenza vaccine uptake among pregnant Black women in Atlanta, Georgia. Positive messaging focused on benefits to infants was found to be important for motivating behaviour change. While trust in social networks was important, the majority of women interviewed placed most importance on receiving a recommendation from their providers (Marsh et al., 2014). In a survey of 1862 pregnant women in Atlanta and Colorado Dudley et al. demonstrated that compared with white women, Black Non-Hispanic and Hispanic women had lower confidence in vaccine safety and efficacy, had lower perceptions of susceptibility to infection and were less likely to report having sufficient knowledge about vaccines (Dudley et al., 2021). In contrast in a study that used PRAMS data from 2004 to 2011 to examine reasons for non-receipt over time in a sample of 8300 women in Georgia, Hispanic patients were more likely to cite fears of harming their infant however there were no differences seen among non-Hispanic Black women when compared with white women for any of the reasons examined (Chamberlain et al., 2016). Outside of the US, several studies identified race as factor impacting vaccine uptake. In the UK, white British women are more likely to be vaccinated or to intend to receive a vaccine during pregnancy than other races (Byrne et al., 2018; Campbell et al., 2015; Carlisle et al., 2019; McAuslane et al., 2018; McQuaid et al., 2018) In Malaysia willingness to be vaccinated against a hypothetical ZIKA vaccine differed by ethnic group (Wong et al., 2017) In France, North African or Asian origin was associated with lack of vaccination against pandemic H1N1 (Freund et al., 2011).
Birthplace and Language Proficiency Language proficiency and birthplace that differed from country of residence were themes that emerged from several countries. Studies from the US, Canada, Australia, Belgium, UK, France, and Israel identified a difference in vaccine coverage or likelihood to receive a vaccine between native borne and non-native born women (Barber et al., 2017; Ben Natan et al., 2017; Cleary et al., 2014; Freund et al., 2011; Krishnaswamy et al., 2018; Laenen et al., 2015; Liu et al., 2012; Maertens et al., 2018). Reasons for these differences in coverage were not explored in these studies. Six studies addressed language proficiency as it related to vaccines. In a study of 5341 women in Washington state, women who spoke either English or Spanish as their first language were more likely than women who spoke another language to have received a H1N1 vaccine (Kay et al., 2012). In New South Wales, Australia, Non-English-speaking women were significantly less likely to have received pertussis vaccination prior to pregnancy or postnatally (Wong et al., 2015). In a qualitative study comparing decision making for receipt of H1N1 vaccine between Scottish national and Polish women living in Scotland, language proficiency emerged an important theme (Sim et al., 2011) In a qualitative study of clinician perspectives on how to improve maternal immunization acceptability in practices in the US, providers described the challenges of working with patients with limited English. Although they recognized that sometimes there may be cultural barriers, they felt that the major barrier is language (Frew et al., 2018).
Provider Recommendation In 152/192 (79%) of studies where the subjects were pregnant women, provider recommendation was identified as an important factor influencing vaccine uptake in pregnancy. A recent metanalysis demonstrated that pregnant women who had received a recommendation for influenza vaccination from a healthcare provider had 12 times higher odds of accepting the vaccine than women who did not. Similarly, the odds of accepting a pertussis vaccine during pregnancy was 10-times greater in women who had received a healthcare provider recommendation (Kilich et al., 2020). Two qualitative studies highlighted that nature of recommendations may differ between providers and by provider type. In a qualitative study which included general practitioners (GPs) and midwives in Northeast London, most health care providers that were interviewed reported mentioning vaccines rather than actively recommending them (Wilson et al., 2019). In a Study in Canada, midwives felt that their role was to inform and not to recommend, they worried that making a recommendation would compromise the principal of informed choice that underpins the Canadian midwifery model of care (Mijović et al., 2020).
Role of the Health Care Provider and Location of Vaccine Delivery
Roles and responsibilities of obstetricians, midwives and GPs in maternal immunization differ according to geographic region. In countries where the model of prenatal care is shared between multiple professionals, a lack of clarity around vaccine roles was frequently identified. A study of 870 providers in Israel identified a lack of clarity in vaccine roles when multiple professionals were involved, lack of ability to store vaccines on site, and lack of time as the three most important barriers to vaccination during pregnancy (Gesser-Edelsburg et al., 2017). Similarly, a qualitative study in Australia identified lack of clarity in vaccine roles, and the need to refer women to their GP to be vaccinated as important barriers to maternal immunization (Webb et al., 2014). The integration of vaccine delivery with prenatal care visits seems to increase uptake in many settings, however the impact may be greater where challenges to accessing healthcare exist. In a study in the UK a greater increase overtime in pertussis vaccine uptake was seen in settings where vaccines were administered in prenatal clinics, however the overall uptake was still higher in settings where GP vaccination continued. The baseline vaccination rates in settings that adopted the new strategy were much lower than in the settings who did not suggesting that point of care delivery of vaccines may have a differential impact on different populations (Llamas et al., 2020). In a study of 842 women in Quito, Ecuador 87% of women who were offered a vaccine at the time of recommendation received a vaccine compared with 15% of women who were recommended but not offered (Erazo et al., 2021). In studies in New York and California, onsite availability of Tdap was associated with increased uptake (New et al., 2018; Wales et al., 2020).
Factors Impacting H1N1 Vaccine Uptake, Lessons Learned from a Pandemic
There were 44 studies included in this review that focused exclusively on factors influencing the uptake of pandemic H1N1 vaccine in pregnancy. Like in non-H1N1 studies, the three major themes of perceptions of the risk and benefit of the vaccine, provider recommendation and access to health care were also dominant during the H1N1 pandemic, and similar disparities in vaccination rates according to race, birthplace and language were observed. Concerns about vaccine safety were expressed by health care providers and patients. In a qualitative study in Australia, women described being actively discouraged to receive the vaccine by their doctors (King et al., 2019). There were, however, some additional themes unique to pandemic H1N1 vaccines that emerged in five qualitative studies with pregnant women (Cassady et al., 2012; Davis et al., 2015; Lohiniva et al., 2014; Lynch et al., 2012; Sim et al., 2011). Anxiety driven by uncertainty was pervasive throughout these studies. Trust in a healthcare provider was strongly emphasized and fear provoked by the media environment was frequently discussed.
Discussion
This systematic narrative review provides a detailed overview of factors impacting uptake of vaccines during pregnancy. The major facilitators of vaccination for pregnant women were provider recommendation and a motivation to protect their infants. The most prominent barriers were lack of a provider recommendation, concerns about vaccine safety, lack of access to prenatal care, and disparities among minority groups and marginalized populations. The review reveals key gaps in the understanding of drivers of vaccine acceptance among pregnant women, especially across different sociodemographic groups. Thus, results can inform efforts to improve uptake of currently recommended vaccines, facilitate acceptance of new vaccines and inform future research activities.
Quantitative, survey-based research was most commonly employed. This methodology is useful for identifying potential factors influencing uptake, however, qualitative and mixed methods research is required to fully understand the reasons behind these factors and to inform interventions. Although a 2015 review from members of the Vaccine Confidence Project called for an increase in qualitative research in this area, qualitative research in this field continues to be underrepresented (Wilson et al., 2015).
Most studies took place in the US, Europe and Australia. There were relatively few studies from low-and middle-income regions, and only fifteen studies from Sub-Saharan Africa. When developing the matrix of determinants of vaccine hesitancy used in this review, the SAGE working group recognised that “the independent and relative strength of influence of each factor is complex and context specific-varying across time, place and vaccines” (Larson et al., 2014). Health systems barriers were likely underrepresented in this review due to the paucity of data from low- and middle-income countries. Additionally, there may be context specific reasons for hesitancy, such as mistrust in western vaccines or government, which were not elicited. New vaccines against infant pathogens such as GBS and RSV are likely to be most impactful in parts of the world where pathogen prevalence is high and screening (GBS) and intensive care (RSV) are not available, however success of any vaccine will be dependent on uptake. Thus, it is imperative that there is more research focused on optimizing maternal immunization in these regions.
Decreased vaccine uptake in marginalized groups was a pervasive theme across geographic regions. This included disparities among racial and ethnic minorities and women whose first language differed from that of their country of residence. The root of these differences is likely to be multifactorial and context specific. Reasons may include language proficiency, lack of trust in a foreign health system, lack of access to pre-natal care and cultural and religious beliefs. In the US, decreased uptake was repeatedly described in Black women. Despite such clear disparities, our review identifies a deficit in studies examining reasons for these differences. The few studies that did explore the reasons for low vaccine uptake among Black women, did so on an individual level. More research that examines the structural reasons behind these disparities is needed. Medical racism is known to impact maternity care in the US, and attitudes towards maternal immunization may be linked to experiences of maternity care but this topic was beyond the scope of this review.
Access to health care was an important theme that overlapped with under immunization of marginalized groups. Lack of access to adequate prenatal care was a more frequently cited factor in studies in low- and middle-income regions, although not unique to these regions. In several studies included in this review, an increased number of prenatal care visits was associated with increased uptake. Given the clear impact of provider recommendation on vaccine uptake, the association between number of prenatal care visits and vaccine uptake may be due to increased opportunity to be recommended and or be offered a vaccine. Access to adequate prenatal care varies across the globe and is lowest in Sub-Saharan Africa and South Asia (UNICEF). In 2018 in the US 6.2% of births were to women who had received no or very late prenatal care (MarchofDimes, 2018). Prenatal care and maternal immunization cannot be separated. Most of the research in this review was hospital or clinic-based rather than population-based. Therefore, the impact of access to prenatal care on vaccine uptake may be underrepresented.
One of the most important factors to impact vaccination of pregnant women in these studies was a recommendation from a health care provider. Research has demonstrated that provider recommendation strongly influences decision making around all vaccines (Smith et al., 2017). Barriers to provider recommendation differed according to geographic regions and provider type.
Most studies focused on attitudinal factors, particularly perceived heuristics. The strongest barrier for pregnant women was safety concerns, within this barrier, safety of their infant was paramount. Similarly, the most prominent motivating factor to receive a vaccine in pregnancy among women was protection of the infant, even in relation to influenza vaccine despite protection of the pregnant woman being the primary goal of administering this vaccine during pregnancy. Messaging to promote maternal vaccination should emphasize benefits for the infant.
The inclusion of studies that took place during the H1N1 pandemic is timely. This review provides an opportunity to reflect on lessons that may help inform the role out of prenatal COVID-19 vaccination. These studies revealed the anxiety provoked by difficult decisions faced by pregnant women and their providers around recommending and receiving a H1N1 vaccine in the absence of robust evidence of vaccine safety and efficacy in pregnant women. A strong recommendation from a trusted healthcare professional was again an important factor influencing uptake. Population-based studies highlighted lower immunization rates in racial and ethnic minority groups and in women whose birthplace differed from their country of residence. Unfortunately, early in the COVID-19 pandemic similar disparities were again seen. Data from the CDC released in May 2021 showed that of a total of 135,968 pregnant women identified in the Vaccine Safety Datalink (VSD) between 14 December 2020 and 8 May 2021, only 11.1% had completed vaccination during pregnancy. The uptake was highest in Asian and white women and lowest in Hispanic and Black women (Razzaghi et al., 2021). In a single centre cohort study in the UK of pregnant women who gave birth between 1 March 2021 and 4 July 2021, 28.7% of eligible pregnant women accepted a COVID-19 vaccine. Uptake was lower in women with younger age, higher levels of deprivation and in women of Afro-Caribbean or Asian ethnicity (Blakeway et al., 2022). This experience in both pandemics highlights the need for radical reform in public health approaches that ensures an early focus on the inclusion of marginalized groups.
This review had limitations. We included only the English language literature and there were very few studies from low- and middle-income countries, potentially limiting the generalizability of the findings. We limited our search to published literature and there may be themes in grey literature that are not included in this review, we opted to include all studies regardless of quality in an effort to understand the breadth of published literature available on this topic While including all studies ensured that a broad range of themes were elicited poorer quality studies may have biased the results. In particular, the results of single centre hospital-based studies which used convenience sampling may not be generalisable. Invalid survey instruments could influence the relative impact of themes elicited. Finally, it should be remembered that in most settings, receiving vaccines during pregnancy is dependent on accessing pre-natal care. The inclusion of the large body of research examining factors that impact accessing prenatal care more generally was beyond the scope of this review.
Conclusion
A strong provider recommendation, equitable access to prenatal care and messaging that focuses on the protection of the infant emerged from the literature as the key components required to achieve good vaccine uptake among pregnant women. These will be important considerations for the introduction of new vaccines, currently in development, against GBS and RSV, both of which will be designed specifically to be given to pregnant women to protect their infants. A relative deficit of qualitative research, research in low- and middle-income regions, research with minority groups and research with prenatal care providers was identified. These areas should be prioritised as the scientific community strives to create maternal vaccines that aim to protect vulnerable infants.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Melanie Cedrone from the Biomedical library at the University of Pennsylvania for her assistance in defining the search terms for this review.
Author Contributions
SG; Conceptualization, Methodology, Investigation, Formal analysis, Writing-Original Draft. SS; Methodology, Validation, Investigation, Writing-Review & Editing. KMB; Conceptualization, Methodology, Writing-review and Editing, Supervision. KAF; Conceptualization, Methodology, Writing-review and editing, Supervision.
Funding
This work was supported by a clinical research fellowship grant from the National Children’s Research Centre, Dublin, Ireland. Grant No. D/19/6 awarded to Sarah Geoghegan.
Declarations
Competing Interests
Kristen Feemster is currently employed by Merck Research laboratories. Employment commenced after conceptualization of this review.
Ethical Approval
This is a review article thus approval from ethics committee was not required.
Consent to Participate
There are no human research participants thus consent was not required.
Consent to Publish
This is a review article. There are no human research participants thus consent was not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anatea MD, Mekonnen TH, Dachew BA. Determinants and perceptions of the utilization of tetanus toxoid immunization among reproductive-age women in Dukem Town, Eastern Ethiopia: A community-based cross-sectional study. BMC International Health and Human Rights. 2018 doi: 10.1186/s12914-018-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LD, Luong L, Rebmann T, Chang JJ. Racial disparities in U.S. maternal influenza vaccine uptake: Results from analysis of Pregnancy Risk Assessment Monitoring System (PRAMS) data, 2012–2015. Vaccine. 2019;37(18):2520–2526. doi: 10.1016/j.vaccine.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Arriola CS, Suntarattiwong P, Dawood FS, Soto G, Das P, Hunt DR, Sinthuwattanawibool C, Kurhe K, Thompson MG, Wesley MG, Saha S, Tinoco YO. What do pregnant women think about influenza disease and vaccination practices in selected countries. Human Vaccines & Immunotherapeutics. 2021 doi: 10.1080/21645515.2020.1851536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Muscoplat MH, Fedorowicz A. Coverage with tetanus, diphtheria, and acellular pertussis vaccine and influenza vaccine among pregnant women—Minnesota, March 2013–December 2014. Morbidity and Mortality Weekly Report (MMWR) 2017;66(2):56–59. doi: 10.15585/mmwr.mm6602a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi RH, Fortner KB, Munoz FM, Roberts J, Gordon JL, Han HH, Glenn G, Dormitzer PR, Gu XX, Read JS, Edwards K, Swamy GK. Maternal immunization: Opportunities for scientific advancement. Clinical Infectious Diseases. 2014;59:S408–S414. doi: 10.1093/cid/ciu708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Natan M, El Kravchenko B, Sakashidlo K, Mor S. What drives pregnant women’s decisions to accept the pertussis vaccine? Applied Nursing Research. 2017;38:60–63. doi: 10.1016/j.apnr.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, Magee LA, O’Brien P, Rezvani A, von Dadelszen P, Khalil A. COVID-19 vaccination during pregnancy: Coverage and safety. American Journal of Obstetrics and Gynecology. 2022;226(2):236 e231–236 e214. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel B, Mahjoub N, Drewniak N, Launay O, Goffinet F. Failure of the vaccination campaign against A(H1N1) influenza in pregnant women in France: Results from a national survey. Vaccine. 2012;30(38):5661–5665. doi: 10.1016/j.vaccine.2012.06.077. [DOI] [PubMed] [Google Scholar]
- Byrne L, Ward C, White JM, Amirthalingam G, Edelstein M. Predictors of coverage of the national maternal pertussis and infant rotavirus vaccination programmes in England. Epidemiology and Infection. 2018;146(2):197–206. doi: 10.1017/s0950268817002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H, Van Hoek AJ, Bedford H, Craig L, Yeowell A-L, Green D, Yarwood J, Ramsay M, Amirthalingam G. Attitudes to immunisation in pregnancy among women in the UK targeted by such programmes. British Journal of Midwifery. 2015;23(8):566–573. doi: 10.12968/bjom.2015.23.8.566. [DOI] [Google Scholar]
- Carlisle N, Seed PT, Gillman L. Can common characteristics be identified as predictors for seasonal influenza vaccine uptake in pregnancy? A retrospective cohort study from a South London Hospital. Midwifery. 2019;72:67–73. doi: 10.1016/j.midw.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Cassady D, Castaneda X, Ruelas MR, Vostrejs MM, Andrews T, Osorio L. Pandemics and vaccines: Perceptions, reactions, and lessons learned from hard-to-reach Latinos and the H1N1 campaign. Journal of Health Care for the Poor and Underserved. 2012;23(3):1106–1122. doi: 10.1353/hpu.2012.0086. [DOI] [PubMed] [Google Scholar]
- CEBM. (2014). Critical appraisal checklist for cross-sectional study. Retrieved from https://www.cebma.org
- Chamberlain AT, Berkelman RL, Ault KA, Rosenberg ES, Orenstein WA, Omer SB. Trends in reasons for non-receipt of influenza vaccination during pregnancy in Georgia, 2004–2011. Vaccine. 2016;34(13):1597–1603. doi: 10.1016/j.vaccine.2016.01.058. [DOI] [PubMed] [Google Scholar]
- Cleary BJ, Rice U, Eogan M, Metwally N, McAuliffe F. 2009 A/H1N1 influenza vaccination in pregnancy: Uptake and pregnancy outcomes—A historical cohort study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;178:163–168. doi: 10.1016/j.ejogrb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Crosby DA, Deleau D, Brophy C, McAuliffe FM, Mahony R. Uptake of the influenza vaccination in pregnancy. Irish Medical Journal. 2016;109(8):449. [PubMed] [Google Scholar]
- Davis MDM, Stephenson N, Lohm D, Waller E, Flowers P. Beyond resistance: Social factors in the general public response to pandemic influenza. BMC Public Health. 2015 doi: 10.1186/s12889-015-1756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HL, Black CL, Ball S, Donahue S, Fink RV, Williams WW, Kennedy ED, Bridges CB, Lu PJ, Kahn KE, Dean AK, Greby SM. Influenza vaccination coverage among pregnant women—United States, 2014–15 influenza season. Morbidity and Mortality Weekly Report (MMWR) 2015;64(36):1000–1005. doi: 10.15585/mmwr.mm6436a2. [DOI] [PubMed] [Google Scholar]
- Ding H, Black CL, Ball S, Donahue S, Izrael D, Williams WW, Kennedy ED, Bridges CB, Lu PJ, Kahn KE, Grohskopf LA, Greby SM. Influenza vaccination coverage among pregnant women—United States, 2013–14 influenza season. Morbidity and Mortality Weekly Report (MMWR) 2014;63(37):816–821. [PMC free article] [PubMed] [Google Scholar]
- Ding H, Black CL, Ball S, Fink RV, Williams WW, Fiebelkorn AP, Lu PJ, Kahn KE, D’Angelo DV, Devlin R, Greby SM. Influenza vaccination coverage among pregnant women—United States, 2016–17 influenza season. Morbidity and Mortality Weekly Report (MMWR) 2017;66(38):1016–1022. doi: 10.15585/mmwr.mm6638a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraivelu K, Boulet SL, Biswas HH, Adams JC, Haddad LB, Jamieson DJ. Predictors of tetanus, diphtheria, acellular pertussis and influenza vaccination during pregnancy among full-term deliveries in a medically underserved population. Vaccine. 2019;37(41):6054–6059. doi: 10.1016/j.vaccine.2019.08.044. [DOI] [PubMed] [Google Scholar]
- Dubé E, Gagnon D, Kaminsky K, Green CR, Ouakki M, Bettinger JA, Brousseau N, Castillo E, Crowcroft NS, Driedger SM, Greyson D, Cook JL. Vaccination against influenza in pregnancy: A survey of Canadian maternity care providers. Journal of Obstetrics and Gynaecology Canada. 2018 doi: 10.1016/j.jogc.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Dudley MZ, Limaye RJ, Salmon DA, Omer SB, O’Leary ST, Ellingson MK, Spina CI, Brewer SE, Bednarczyk RA, Malik F, Frew PM, Chamberlain AT. Racial/ethnic disparities in maternal vaccine knowledge, attitudes, and intentions. Public Health Reports. 2021 doi: 10.1177/0033354920974660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erazo CE, Erazo CV, Grijalva MJ, Moncayo AL. Knowledge, attitudes and practices on influenza vaccination during pregnancy in Quito, Ecuador. BMC Public Health. 2021;21(1):72. doi: 10.1186/s12889-020-10061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JA, Baltrons R, Rowley E, Quintanilla I, Crespin E, Ropero AM, Ortiz JR, Lambach P, Neuzil KM, Stepanchak M, Hombach J, Bhat N. Implementation of maternal influenza immunization in El Salvador: Experiences and lessons learned from a mixed-methods study. Vaccine. 2018;36(28):4054–4061. doi: 10.1016/j.vaccine.2018.05.096. [DOI] [PubMed] [Google Scholar]
- Frawley JE, McKenzie K, Sinclair L, Cummins A, Wardle J, Hall H. Midwives’ knowledge, attitudes and confidence in discussing maternal and childhood immunisation with parents: A national study. Vaccine. 2020;38(2):366–371. doi: 10.1016/j.vaccine.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Freund, R., Le Ray, C., Charlier, C., Avenell, C., Truster, V., Tréluyer, J. M., Skalli, D., Ville, Y., Goffinet, F., Launay, O., & Inserm COFLUPREG Study Group Determinants of non-vaccination against pandemic 2009 H1N1 influenza in pregnant women: A prospective cohort study. PLoS ONE. 2011 doi: 10.1371/journal.pone.0020900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew PM, Randall LA, Malik F, Limaye RJ, Wilson A, O’Leary ST, Salmon D, Donnelly M, Ault K, Dudley MZ, Fenimore VL, Omer SB. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Human Vaccines & Immunotherapeutics. 2018;14(7):1548–1557. doi: 10.1080/21645515.2018.1425116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesser-Edelsburg A, Shir-Raz Y, Hayek S, Aassaraf S, Lowenstein L. Despite awareness of recommendations, why do health care workers not immunize pregnant women? American Journal of Infection Control. 2017;45(4):436–439. doi: 10.1016/j.ajic.2016.11.025. [DOI] [PubMed] [Google Scholar]
- Heath PT, Culley FJ, Jones CE, Kampmann B, Le Doare K, Nunes MC, Sadarangani M, Chaudhry Z, Baker CJ, Openshaw PJM. Group B streptococcus and respiratory syncytial virus immunisation during pregnancy: A landscape analysis. Lancet Infectious Diseases. 2017;17(7):e223–e234. doi: 10.1016/S1473-3099(17)30232-3. [DOI] [PubMed] [Google Scholar]
- Howland R. Influenza vaccination among pregnant women—Massachusetts, 2009–2010. Morbidity & Mortality Weekly Report (MMWR) 2013;62(43):854–857. [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Ali I, Ekmekcioglu C, Kundi M. Increasing frequency of antenatal care visits may improve tetanus toxoid vaccination coverage in pregnant women in Pakistan. Human Vaccines & Immunotherapeutics. 2020;16(7):1529–1532. doi: 10.1080/21645515.2019.1705693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MK, Koelemay KG, Kwan-Gett TS, Cadwell BL, Duchin JS. 2009 Pandemic influenza a vaccination of pregnant women: King County, Washington State, 2009–2010. American Journal of Preventive Medicine. 2012;42(6 SUPPL. 2):S172–S179. doi: 10.1016/j.amepre.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kennedy ED, Ahluwalia IB, Ding H, Lu PJ, Singleton JA, Bridges CB. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. American Journal of Obstetrics and Gynecology. 2012;207(3 SUPPL.):S9–S16. doi: 10.1016/j.ajog.2012.06.069. [DOI] [PubMed] [Google Scholar]
- Kilich E, Dada S, Francis MR, Tazare J, Chico RM, Paterson P, Larson HJ. Factors that influence vaccination decision-making among pregnant women: A systematic review and meta-analysis. PLoS ONE. 2020;15(7):e0234827. doi: 10.1371/journal.pone.0234827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CL, Chow MY, Leask J, Wiley KE. Australian caregivers’ perceptions of influenza vaccination in pregnancy: A mixed methods exploration. Women and Birth. 2019;32(3):240–245. doi: 10.1016/j.wombi.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Koerner J, Forinash AB, Yancey AM, Brinkmeyer J, Dingman S, Miller C, Thompson J, Bergin L, López JD, Ravin A. Administration rates of the Tdap vaccine in obstetric patients. Annals of Pharmacotherapy. 2018;52(7):655–661. doi: 10.1177/1060028018755454. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Cheng AC, Wallace EM, Buttery J, Giles ML. Understanding the barriers to uptake of antenatal vaccination by women from culturally and linguistically diverse backgrounds: A cross-sectional study. Human Vaccines & Immunotherapeutics. 2018;14(7):1591–1598. doi: 10.1080/21645515.2018.1445455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laenen J, Roelants M, Devlieger R, Vandermeulen C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine. 2015;33(18):2125–2131. doi: 10.1016/j.vaccine.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- Liu N, Sprague AE, Yasseen IAS, Fell DB, Wen SW, Smith GN, Walker MC. Vaccination patterns in pregnant women during the 2009 H1N1 influenza pandemic: A population-based study in Ontario, Canada. Canadian Journal of Public Health. 2012;103(5):353–358. doi: 10.1007/BF03404440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas A, Amirthalingam G, Andrews N, Edelstein M. Delivering prenatal pertussis vaccine through maternity services in England: What is the impact on vaccine coverage? Vaccine. 2020;38(33):5332–5336. doi: 10.1016/j.vaccine.2020.05.068. [DOI] [PubMed] [Google Scholar]
- Lohiniva AL, Barakat A, Dueger E, Restrepo S, El Aouad R. A qualitative study of vaccine acceptability and decision making among pregnant women in Morocco during the A (H1N1) pdm09 pandemic. PLoS ONE. 2014 doi: 10.1371/journal.pone.0096244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MM, Mitchell EW, Williams JL, Brumbaugh K, Jones-Bell M, Pinkney DE, Layton CM, Mersereau PW, Kendrick JS, Medina PE, Smith LR. Pregnant and recently pregnant women’s perceptions about influenza A pandemic (H1N1) 2009: Implications for public health and provider communication. Maternal and Child Health Journal. 2012;16(8):1657–1664. doi: 10.1007/s10995-011-0865-y. [DOI] [PubMed] [Google Scholar]
- MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Maertens K, Braeckman T, Blaizot S, Theeten H, Roelants M, Hoppenbrouwers K, Leuridan E, Van Damme P, Vandermeulen C. Coverage of recommended vaccines during pregnancy in Flanders, Belgium. Fairly good but can we do better? Vaccine. 2018;36(19):2687–2693. doi: 10.1016/j.vaccine.2018.03.033. [DOI] [PubMed] [Google Scholar]
- MarchofDimes. (2018). Retrieved from https://www.marchofdimes.org/peristats/ViewTopic.aspx?reg=99&top=5&lev=0&slev=1
- Marsh HA, Malik F, Shapiro E, Omer SB, Frew PM. Message framing strategies to increase influenza immunization uptake among pregnant African American women. Maternal and Child Health Journal. 2014;18(7):1639–1647. doi: 10.1007/s10995-013-1404-9. [DOI] [PubMed] [Google Scholar]
- McAuslane H, Utsi L, Wensley A, Coole L. Inequalities in maternal pertussis vaccination uptake: A cross-sectional survey of maternity units. Journal of Public Health (Oxford, England) 2018;40(1):121–128. doi: 10.1093/pubmed/fdx032. [DOI] [PubMed] [Google Scholar]
- McQuaid F, Jones C, Stevens Z, Meddaugh G, O'Sullivan C, Donaldson B, Hughes R, Ford C, Finn A, Faust SN, Gbesemete D, Snape MD. Antenatal vaccination against Group B streptococcus: Attitudes of pregnant women and healthcare professionals in the UK towards participation in clinical trials and routine implementation. Acta Obstetricia et Gynecologica Scandinavica. 2018;97(3):330–340. doi: 10.1111/aogs.13288. [DOI] [PubMed] [Google Scholar]
- Mendoza-Sassi RA, Cesar JA, Cagol JM, Duarte IA, Friedrich LM, dos Santos VK, Zhang LJ. 2010 A(H1N1) vaccination in pregnant women in Brazil: Identifying coverage and associated factors. Cadernos De Saude Publica. 2015;31(6):1247–1256. doi: 10.1590/0102-311x00084514. [DOI] [PubMed] [Google Scholar]
- Mendoza-Sassi RA, Linhares AO, Schroeder FMM, Maas NM, Nomiyama S, César JA. Vaccination against influenza among pregnant women in southern Brazil and associated factors. Ciência & Saúde Coletiva. 2019;24(12):4655–4664. doi: 10.1590/1413-812320182412.08382018. [DOI] [PubMed] [Google Scholar]
- Merritt TA, Rasmussen SA, Bright MA, Roussos-Ross D, Sims SM, Gurka MJ, Thompson LA. Variation in Tdap and influenza vaccination coverage among pregnant women by insurance type—Florida, 2016–2018. Morbidity and Mortality Weekly Report (MMWR) 2020;69(3):72–76. doi: 10.15585/mmwr.mm6903a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijović H, Greyson D, Gemmell E, Trottier M, Vivion M, Graham JE, Dubé È, Bettinger JA. Perinatal health care providers’ approaches to recommending and providing pertussis vaccination in pregnancy: A qualitative study. CMAJ Open. 2020;8(2):E377–e382. doi: 10.9778/cmajo.20190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniz MH, Beigi RH. Maternal immunization clinical experiences, challenges, and opportunities in vaccine acceptance. Human Vaccines & Immunotherapeutics. 2014;10(9):2562–2570. doi: 10.4161/21645515.2014.970901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New S, Winter K, Boyte R, Harriman K, Gutman A, Christiansen A, Royce S. Barriers to receipt of prenatal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine among mothers of infants aged < 4 months with pertussis—California, 2016. Morbidity and Mortality Weekly Report (MMWR) 2018;67(38):1068–1071. doi: 10.15585/mmwr.mm6738a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JY, Seo YB, Song JY, Choi WS, Lee J, Jung E, Kang S, Choi MJ, Jun J, Yoon JG, Lee SN, Kim WJ. Perception and attitudes of Korean obstetricians about maternal influenza vaccination. Journal of Korean Medical Science. 2016;31(7):1063–1068. doi: 10.3346/jkms.2016.31.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes J, Booth A, Flemming K, Garside R, Harden A, Lewin S, Pantoja T, Hannes K, Cargo M, Thomas J. Cochrane Qualitative and Implementation Methods Group guidance series-paper 3: Methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. Journal of Clinical Epidemiology. 2018;97:49–58. doi: 10.1016/j.jclinepi.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Omer SB. Maternal immunization. New England Journal of Medicine. 2017;376(13):1256–1267. doi: 10.1056/NEJMra1509044. [DOI] [PubMed] [Google Scholar]
- Patel MK, Goodson JL, Alexander JP, Jr, Kretsinger K, Sodha SV, Steulet C, Gacic-Dobo M, Rota PA, McFarland J, Menning L, Mulders MN, Crowcroft NS. Progress toward regional measles elimination—worldwide, 2000–2019. Morbidity and Mortality Weekly Report (MMWR) 2020;69(45):1700–1705. doi: 10.15585/mmwr.mm6945a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaghi H, Kahn KE, Black CL, Lindley MC, Jatlaoui TC, Fiebelkorn AP, Havers FP, D’Angelo DV, Cheung A, Ruther NA, Williams WW. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. Morbidity and Mortality Weekly Report (MMWR) 2020;69(39):1391–1397. doi: 10.15585/mmwr.mm6939a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, Tat’Yana AK, Lamias MJ, Irving SA, Kauffman TL, Vesco KK, Patel SA. COVID-19 vaccination coverage among pregnant women during pregnancy—eight integrated health care organizations, United States, December 14, 2020–May 8, 2021. Morbidity and Mortality Weekly Report (MMWR) 2021;70(24):895–899. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan AK, Mak DB, Hauck YL, Gibbs R, Tracey L, Effler PV. Trends in seasonal influenza vaccine uptake during pregnancy in Western Australia: Implications for midwives. Women and Birth. 2016;29(5):423–429. doi: 10.1016/j.wombi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Sim JA, Ulanika AA, Katikireddi SV, Gorman D. ‘Out of two bad choices, I took the slightly better one’: Vaccination dilemmas for Scottish and Polish migrant women during the H1N1 influenza pandemic. Public Health. 2011;125(8):505–511. doi: 10.1016/j.puhe.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Smith LE, Amlot R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35(45):6059–6069. doi: 10.1016/j.vaccine.2017.09.046. [DOI] [PubMed] [Google Scholar]
- UNICEF. https://data.unicef.org/topic/maternal-health/antenatal-care/
- Vishram B, Letley L, Jan Van Hoek A, Silverton L, Donovan H, Adams C, Green D, Edwards A, Yarwood J, Bedford H, Amirthalingam G, Campbell H. Vaccination in pregnancy: Attitudes of nurses, midwives and health visitors in England. Human Vaccines & Immunotherapeutics. 2018;14(1):179–188. doi: 10.1080/21645515.2017.1382789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales DP, Khan S, Suresh D, Ata A, Morris B. Factors associated with Tdap vaccination receipt during pregnancy: A cross-sectional study. Public Health. 2020;179:38–44. doi: 10.1016/j.puhe.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Webb H, Street J, Marshall H. Incorporating immunizations into routine obstetric care to facilitate Health Care Practitioners in implementing maternal immunization. Human Vaccines and Immunotherapeutics. 2014;10(4):1114–1121. doi: 10.4161/hv.27893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. 2015;33(47):6420–6429. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- Wilson R, Paterson P, Larson HJ. Strategies to improve maternal vaccination acceptance. BMC Public Health. 2019;19(1):342. doi: 10.1186/s12889-019-6655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CY, Thomas NJ, Clarke M, Boros C, Tuckerman J, Marshall HS. Maternal uptake of pertussis cocooning strategy and other pregnancy related recommended immunizations. Human Vaccines & Immunotherapeutics. 2015;11(5):1165–1172. doi: 10.1080/21645515.2015.1019188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, L. P., Alias, H., Hassan, J., & AbuBakar, S. (2017). Attitudes towards Zika screening and vaccination acceptability among pregnant women in Malaysia.Vaccine, 35(43), 5912–5917. 10.1016/j.vaccine.2017.08.074 [DOI] [PubMed]
- Yaya S, Kota K, Buh A, Bishwajit G. Antenatal visits are positively associated with uptake of tetanus toxoid and intermittent preventive treatment in pregnancy in Ivory Coast. BMC Public Health. 2019;19(1):1467. doi: 10.1186/s12889-019-7847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaya S, Kota K, Buh A, Bishwajit G. Prevalence and predictors of taking tetanus toxoid vaccine in pregnancy: A cross-sectional study of 8,722 women in Sierra Leone. BMC Public Health. 2020;20(1):855. doi: 10.1186/s12889-020-08985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.