Abstract
This study extracted ellagitannins from rambutan peel using the Soxhlet technique. The extract was further partitioned and fractionated to get extract rich in ellagitannin and geraniin, respectively. The partitioning of the extract significantly increased total phenolic content (TPC) by 36.3% and its biological properties. Mineral elements such as Ca, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, and Zn were identified in both peel and extract. Ellagitannins such as geraniin and corilagin with metabolites (gallic acid and ellagic acid) were identified as the major compounds. Analysis of antioxidant activities shows that the ellagitannin rich extract is as powerful as vitamin C. Geraniin was the main contributor to the free radical scavenging activity. The study also revealed that extract with a fraction rich in geraniin has antioxidant activity equivalent to commercial geraniin (1.56 ± 0.11 Trolox equivalent g/g). It also showed low cytotoxicity on fibroblast L929 cells, moderate tyrosinase activity, and good efficacy against Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes strains. Successive fractionation of the extract is a promising technique to produce geraniin rich fractions with enhanced antioxidant property. Rambutan peel, as a natural product, is a good source of mineral elements and biologically active compounds for pharmaceutical, nutraceutical, and cosmetic formulations.
1. Introduction
Natural products (secondary metabolites) from plants are organic compounds in the correct chiral configuration to exert biological activity, however not directly involved in the primary function such as normal growth, development, or reproduction of the plant.1 They provide defense mechanisms against various biotic and abiotic factors2,3 and are relatively small molecules that exhibit structural and chemical diversity.1,4 They are mostly located in the outer layers of plant tissue due to their protective roles. Secondary metabolites can be broadly classified into simple phenolic acids, terpenes, flavonoids, xanthones, stilbenes, and lignans or more complex polyphenols which include condensed and hydrolyzable tannins.5 These compounds are potentially active ingredients in prescription and nonprescription drugs (pharmaceuticals) and cosmetics (cosmeceuticals) as well as dietary supplements and natural health products (nutraceuticals).1 Antimicrobial and antibiofilm activities of plant-derived phenolic compounds against an array of microorganisms, as well as low toxicity and safety, have been reported.6 Antimicrobial properties of specifically hydrolyzable tannins are well established and attributed to the presence of hexahydroxydiphenoyl and nonahydroxyterphenoyl moieties.7−9 They exert different mechanisms of action on microorganisms, including direct interference with microbial metabolism, adhesion capacity, enzymatic inhibition, removal of essential substances, metal ions complexations, and destruction of membrane cells.6 Hydrolyzable tannins such as ellagitannins also exert pharmacological effects, including free radical scavenging activity (antioxidant), antinutritional and cardio-protective properties,10,11 antilaryngeal cancer activity in vitro and in vivo,12 modulation of gut diseases,13,14 anticancer,14,15 antihypertensive,16 and many others.
In cosmetics, ellagitannins are reported to ameliorate oxidative stress of the primary cell in the epidermis (keratinocyte) by increasing glutathione levels and cell viability.17 Tyrosinase (TYR) is an enzyme responsible for melanin synthesis in conjunction with TYR-related protein 1 (TYRP1) and dopachrome tautomerase (TYRP2) which transform melanin into eumelanine and phoemelanine to protect skin from harmful ultraviolet (UV) damage.18−20 However, excess melanin production by TYR causes hyperpigmentation. The current skin-whitening agents for improving skin pigmentation such as hydroquinone (benzene-1,4-diol or quinol), a derivative of benzene and antimelanogenic agents including retinol, are characterized by high toxicity, burning, redness, allergy, and cancer.20,21 Researchers have been focusing on developing natural whitening agents with selective activity on TYR to reduce hyperpigmentation without toxicity to normal and healthy cells.19 Naturally occurring hydrolyzable tannins such as coumaric acid and caffeic acid were reported to have inhibited melanin production with no significant cytotoxicity.20 Pomegranate extract also inhibited tyrosinase activity and melanin production.22 Furthermore, several health benefits are epidemiologically attributed to the consumption of plant-derived foods due to phytochemical constituents and polyphenols.23,24 This has brought the concept of nutraceutical and natural food additives. Nutraceuticals are bioactive phytochemicals/compounds containing foods in pharmaceutical forms as pills, powders, capsules, and vials with nutritional benefits and medicinal effects.25 Studies on bread enriched with phytochemicals were reported to enhance sensory attributes, shelf life, and nutrient content.26,27 These health promoting metabolites are predominantly found in various types of fruits and vegetables.
Rambutan (Nephelium lappaceum L. (N. lappaceum)) is a tropical fruit in the family of Sapindaceae and an exotic fruit indigenous to Southeast Asia. It is cultivated in different parts of the world, with Thailand, Indonesia, and Malaysia as the main producing countries.27 The edible and succulent fruit is rich in sugars, organic acids, minerals, dietary fiber, and vitamin C.28−31 About 270000 tons of rambutan fruits are produced in Thailand every year and processed into various forms. Out of this, the peel/waste contributes to 45.9–64.7% of the total weight.27,32 The peel is a rich source of mineral elements and ellagitannins that could be put into best use to reduce waste via economically viable processes and procedures to produce value-added products.
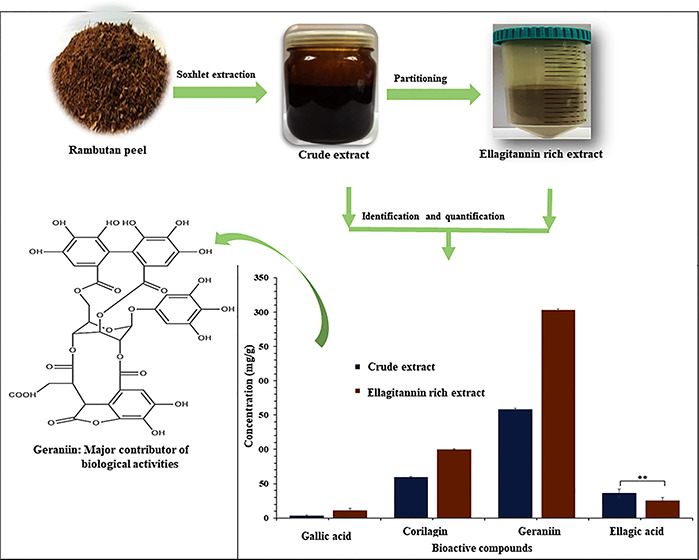
Currently, there has been an increasing global demand for bioactive compounds from plants by the pharmaceutical, cosmetic, and nutraceutical industries, as a result of their presumed roles in disease prevention, nutritional value, and ability to address human skin-related issues.33 Studies have shown that extracts from rambutan peel possess ellagitannins that exhibit various biological activities in humans.31,34 Among the ellagitannins, geraniin is the major compound in rambutan peel.27 Geraniin is a substance that possesses a broad range of biological activities. Some of these include cytoprotective, neuroprotective antiulcer, antioxidant, antiviral, antimicrobial, prevention and treatment of osteoporosis, tissue regeneration, stimulation of dermal fibroblasts and keratinocytes, enhanced glucose uptake, immune modulation, analgesic properties, antihyperglycemic, protection against liver damage, and cardiovascular disease and metabolic dysregulation, and many others.35−39 The natural occurrence of geraniin and the variety of its biological properties make it a suitable candidate for pharmaceutical, nutraceutical, cosmetic, and industrial applications. However, separation of this compound from a natural source has been a major challenge due to structural resemblance in ellagitannins.
Considering the global demand for natural mineral supplements and bioactive compounds from plants, this study takes a holistic look at rambutan peel and its extractive as a potential material for meeting these demands. The possibility of essential mineral elements in the peel, biological activity of the extract, and a more efficient method for separating geraniin were evaluated. This study will contribute greatly to the existing knowledge in the field of utilizing rambutan peel in pharmaceutical, nutraceutical, cosmetic, and other industries.
2. Materials and Methods
2.1. Materials
Rambutan (N. lappaceum) is harvested between May and July every year. The peels were obtained from Malee Sampran Public Co., Ltd., Nakhon Pathom Province (central province of Thailand), in May 2019. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) were purchased from Sigma-Aldrich, USA. Commercial grade 95% ethanol was bought from the Liquor Distillery Organization (LDO), Thailand. All other reagents were of analytical grade and used as received without any further purification.
2.2. Extraction of BCs from Rambutan Peel
Dried rambutan peel with a mass of 400 g was placed in a woven sack which was stacked with cotton wool before being placed into the main chamber of the Soxhlet apparatus. The extraction solvent (95% ethanol) with a substrate to solvent ratio of 1:10 was continuously cycled through the matrix. The Soxhlet apparatus was equipped with a condenser, and the solvent was heated to 78 °C. The boiling and reflux of the solvent continued for 8 h. The process was repeated (3 times) until the solvent around the sample appeared clean. The solvent containing the extract was transferred into a round-bottom flask for further processing.
2.3. Determination of Extract Yield
The extract was filtered using grade 1 Whatman qualitative filter paper and evaporated to remove solvent using a Laborata 4000 rotary evaporator (Heidolph, Japan) operated at 40 °C. Thereafter, the crude extract was further partitioned using ethyl acetate to remove some nonpolar constituents and impurities. The partitioning was carried out with modifications from a previous method.40 20 g of crude extract was dissolved in 250 mL of ultrapure water and mixed with 250 mL of ethyl acetate in a separating funnel. The mixture was shaken, and the ethyl acetate fraction was collected, filtered, and evaporated afterward to get the ellagitannin rich extract. The extract was weighed with an analytical balance to determine the percentage yield. The yields of crude and partition extracts were determined using the equation below;
| 1 |
2.4. Analysis of Total Phenolic Content (TPC)
The total phenolics contents of the extracts were determined using Folin–Ciocalteau Reagent (FCR) as described by ref (41) with modifications. In brief, 125 μL of diluted sample was mixed with 500 μL of reverse osmosis (RO) water and 125 μL of FCR in the initial step. After 6 min, 1.25 mL of 7% (w/v) sodium carbonate was added to the reaction followed by1000 μL of RO water to dilute the mixture to 3 mL before incubation for 90 min at room temperature. The absorbance was measured at 760 nm using a UV–vis spectrophotometer (UVmini-1240, Shimadzu, Japan). Standard solutions of gallic acid with concentrations in the range of 60–300 μg/mL wer used to prepare a standard curve.42 The total phenolic content of the extracts was expressed as micrograms (μg) of gallic acid equivalent (GAE) per milligram (mg) of dried sample.
2.5. Analysis of Minerals Element and Pesticide Residue in Rambutan Peel
The mineral element content of the rambutan peel and ellagitannin rich extract (which is of interest in this study) was determined using inductively coupled plasma optical emission spectroscopy (ICP-OES; Optima 8300, PerkinElmer, Singapore) with Syngistix for ICP, version 2.0 software to analyze the elements. Instrument calibration standard 2 (PerkinElmer Plus, USA) was used as the standard material, and microwave digestion was carried out with (Titan MPS, PerkinElmer, Germany). The sample (0.4008 g) was digested in 7.0 mL of HNO3 and 3.0 mL of H2O2. Thereafter, the solution was filtered using filter paper grade 1 (no. 1) and diluted to 100.00 mL with type 1 water. The digestion was done with a three-step process at temperatures of 170, 190, and 50 °C and holding times of 5, 30, and 15 min, respectively. The pesticide residue test of rambutan peel was carried out following in-house method TE-CH-032.43
2.6. Antioxidant Activity Assays
2.6.1. 2,2-Diphenyl-2-picrlhydrazyl Radical Scavenging Activity (DPPH)
The free radical scavenging antioxidant activity of the extracts was analyzed by DPPH assay. DPPH assay was evaluated with some modifications from ref (42). The extracts (0.1 mg/mL) were diluted to five different concentrations, and 1 mL of each concentration was mixed with 1 mL of 0.1 mM DPPH in 95% ethanol and incubated in the dark at room temperature for 30 min. The absorbance was measured at 517 nm using a UV–vis spectrophotometer (UVmini-1240 Shimadzu, Japan). Butylhydroxytoluene (BHT), α-tocopherol (vitamin E), and ascorbic acid (vitamin C) were used as positive control. The antioxidant activity was calculated as the radical scavenging effect (%) using eq 2. Half-maximal inhibitory concentration (IC50) values were calculated from the plot of radical scavenging effect percentages against sample concentration, where IC50 is the concentration of sample at which 50% of free radical scavenging activity occur.
| 2 |
where A0 is absorbance of the standard and A1 is the absorbance of the sample. IC50 values were calculated from the plot of radical scavenging effect percentages against sample concentration, where IC50 is the concentration of sample at which 50% of free radical scavenging activity occurs.
2.6.2. 2,3-Ethylbenzothiazoline-6-sulfonic Acid Radical Scavenging Activity (ABTS)
Antioxidant activity with ABTS assay was carried out according to the method of.44 The ABTS•+ radical cation solution was prepared by mixing 7 mM ABTS reagent with 2.45 mM potassium persulfate (K2S2O8) and stored in the dark for 12 h. Thereafter, dilution was done to obtain an absorbance around 0.70 ± 0.02 at wavelength of 743 nm. Two mL of the solution was mixed with 20 μL of diluted sample and incubated for 6 min before measuring the absorbance by UV–vis spectrophotometer (UVmini-1240 Shimadzu, Japan) at wavelength of 734 nm. Each measurement was performed in triplicate. The positive control is comprised of BHT, α-tocopherol (vitamin E), and ascorbic acid (vitamin C). The percent of ABTS•+ radical scavenging activity was calculated using eq 2.
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
The FRAP reagent was prepared by mixing 10 μL of acetate buffer (300 mM), 1 mL of FeCl3·6H2O (20 μM), 1 mL of TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) solution in HCl (40 μM), and 1.2 mL of deionized water. The antioxidant activity was measured using a reducing agent. Briefly, 60 μL of diluted samples, 180 μL of deionized water, and 1.8 mL of FRAP reagent were mixed and incubated at 37 °C for 4 min to perform the reaction. The absorbance of each solution was recorded at 595 nm. The antioxidant capacity was calculated using the standard curve from standard solutions (ferrous sulfate at various concentrations from 0.1 to 1.0 mM). The antioxidant capacities of the samples were expressed as μM Fe(II)/g of sample.27 Butylhydroxytoluene (BHT), α-tocopherol (vitamin E), and ascorbic acid (vitamin C) were used as positive control.
2.7. Identification and Quantification of Polyphenolic Compounds by HPLC
The main polyphenols in the extracts (gallic acid, corilagin, geraniin, and ellagic acid) were quantified using a high performance liquid chromatographer (HPLC; Waters, USA) equipped with an Inertsil ODS-3 C18 (4.6 mm × 150 mm, 5 μm) column.27 The experiment was conducted using a gradient elution of 0.4% formic acid in methanol with a flow rate of 1.0 mL/min and a column temperature of 30 °C. The peaks of the sample were measured at 280 nm using a variable wavelength detector. The amount of the extract was calculated from the known standard calibration curves of the compounds.
2.8. MTT Cytotoxicity Assay
Cytotoxicity analysis of peel and extract was carried out on the basis of ISO 10993-5 standard. The cellular response of the test specimen extract was evaluated using a 3-(4, 5-dimethythiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay. Minimum Essential Medium using Mouse fibroblast L929 cells (Mouse Fibroblast Cells, ATCC CCLI, NCTC 929, of Stain L) was used to perform indirect cytotoxicity. A 96-well plate at cell suspension of 1 × 105 cells/mL of L929 was seeded with cells and incubated for 24 ± 2 h at 5 ± 0.1% CO2, 37 ± 1 °C, and 95 ± 5% relative humidity to obtain a confluent monolayer of cells before testing. The specimens with mass-to-volume ratio of 0.1 mg/mL were sterilized using ethylene oxide gas and extracted at 37 ± 1 °C for 24 ± 2 h. The extract was then filtered using a 0.22 μm syringe filter before testing. After further incubation for 24 ± 2 h, the viable cells were stained with MTT and incubated for an extra 2 h. DMSO was added to each well after the MTT was removed, and the absorbance was measured using a microplate reader (EASYS, UVM340 S/N ASY54180) at 570 nm. The polyurethane film containing 0.1% zinc diethyldithiocarbamate (ZDEC):RM-A was used as positive control material and Thermanox (Nunc) coverslip for negative control.45
2.9. Fractionation and Confirmation of Major Ellagitannins in Extract
The partitioned ethanolic rambutan peel extract of a 10 g admixture with 100 g of silica and 100 mL of mobile phase was subjected to quick column chromatography on silica packed and eluted with a mixture of dichloromethane and methanol in the ratio of 80:20 as the mobile phase. The mobile phase was continually added to the top of the column with the solvent flow at a rate of 25 mL/min, which continued through the column and collected in a beaker. The fractions were concentrated by evaporation of solvent under nitrogen gas at room temperature. The various fractions were identified by the method described in Section 2.7 (HPLC).
2.10. Antioxidant Activity Contribution of Extracts
Trolox equivalent DPPH free radical scavenging antioxidant capacity of the various fractions of extract were evaluated by using DPPH assay. A standard calibration curve was prepared using Trolox. A 0.1 mg/mL concentration of Trolox and samples were prepared, and 600 μL of 0.2 mmol of DPPH solution was transferred into test tube and diluted with 50% ethanol at different volumes of (600 – a) μL (a = volume of sample, at 0, 30, 60, 120, or 240 μL). The mixture was incubated for 5 min before the absorbance was measured at 520 nm. The sample was expressed as gram of Trolox equivalent antioxidant capacity (TEAC) per gram of sample.27
2.11. Analysis of Tyrosinase Activity
The tyrosinase inhibitory activity of extract was carried out by modifying the methods reported by refs (46 and 47). In brief, 1800 μL of 0.1 M sodium phosphate buffer was added to 1000 μL of l-3,4-dihydroxyphenylalanine (l-DOPA) as substrate for the enzyme and left to react for 10 min. After 10 min, 100 μL of sample (dissolved in dimethyl sulfoxide (DMSO) and diluted to different concentrations) was added. This was followed immediately by 100 μL of mushroom tyrosinase (138 unit/ml) and left in the dark for 10 min. The solution was homogeneously mixed, and the absorbance was measured at 475 nm using a UV–vis spectrophotometer (UVmini-1240 Shimadzu, Japan). Kojic acid was used as positive control. The concentration at which half of the original tyrosinase activity was inhibited (IC50) was calculated using eq 3.
| 3 |
where A = absorbance of control, without test sample; B = absorbance of sample, with tyrosinase; and C = absorbance of sample blank, without tyrosinase, where IC50 is the concentration of the sample at which 50% tyrosinase inhibition occurred.
2.12. In Vitro Antimicrobial Activity of Extract
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of rambutan peel extract were determined in 96-well microplates using broth microdilution method.48 Mueller–Hinton broth was used as diluent for aerobic Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis) strains and an aerotolerant anaerobe Cutibacterium acnes (C. acnes) strain. The diluent and bacterial growth were used as positive control, while the negative control contained only bacteria strains. Microplates containing aerobic strains were inoculated at 37 °C for 24 h, while the aerotolerant anaerobe strain was incubated in an aerobic container for 74 h. To determine the MBC, 10 μL of bacterial suspension was removed from each well after overnight growth (before adding the resazurin working solution) and spread onto the Mueller–Hinton agar prior to incubation at 37 °C for 24 h. The MBC was defined as the lowest concentration of extract at which 99.9% of the inoculated microorganisms were killed.49
2.13. Statistical Analysis
The experiments were performed in triplicate. A one-way analysis of variance (ANOVA) was used for comparing mean values with statistical significance at p < 0.05. A Bonferroni multiple range test was used for comparing group means.
3. Results and Discussion
3.1. Percentage Yield and Total Phenolic Content of Extracts
Soxhlet extraction is considered as an exhaustive extraction technique for high spectrum recovery of compounds that are thermally stable. The results in Table 1 show the yields of crude ethanolic extract and ellagitannin rich extract. There was high recovery of 38.8 ± 1.7% bioactive compounds from the rambutan peels compared with previous studies.27,50,51 Further separation of active compound in ethyl acetate gave a yield of 12.9 ± 0.9%. The partitioning resulted in highly significant increase in TPC of 713.4 ± 21.9 mg of GAE/g as compared with the crude extract of 454.4 ± 39.1 mg of GAE/g. The increase in TPC could be explained as a result of the removal of nonphenolic compounds and compounds such as carbohydrates, water-soluble vacuolar pigments, and other impurities, which contributed to about 67.8% of the total yield.
Table 1. Yield and Total Phenolic Content of Crude and Ellagitannin Rich Extracts from Soxhlet.
| sample | yield (%) | TPC (mg of GAE/g) |
|---|---|---|
| crude extract | 38.8 ± 1.7 | 454.4 ± 39.1 |
| ellagitannin rich extract | 12.9 ± 0.9 | 713.4 ± 21.9 |
3.2. Mineral Elements and Pesticide Residue in Rambutan Peel
Rambutan is considered as the hub for bioactive compounds which play a biological importance in the plant. However, little has been said about the mineral composition of the peel for consideration as additive in feed and food. Table 2 shows the mineral compositions of rambutan peel determined by ICP-OES. Mineral elements such as Ca, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, and Zn were found in the peel and ellagitannin rich extract, respectively. Among the various elements, K, Ca, and Mg were in high concentrations in the raw peels, while K, Ca, and Na were high in the ellagitannin rich extract. The reduction in the minerals concentration in the ellagitannin rich extract is undoubtedly a result of the extraction process, which was unable to recover all of the minerals. In order to validate the appropriateness of the peel to serve as dietary supplement, the possible pesticide residue was analyzed in a preliminary study (data not shown). The carbamate, organochlorine, organophosphate, and pyrethroid groups were analyzed with no traces of their residue in the peel except for the pyrethroid group, which shows the presence of lead (Pb) around <0.025 mg/kg. The Pb residue in the peels may either be absorbed from airborne deposit on the exposed rambutan fruits or through the root system from polluted environments.52 Interestingly, the Pb content in the rambutan peel did not exceed the acceptable limit of 1 mg/kg set by the Thai Ministry of Public Health53 and Codex Alimentarius guidelines by the World Health Organization (WHO)/Food and Agriculture Organization (FAO) of 0.1 mg/kg.54,55 Considering the high mineral contents of the peel, it could serve as a good source of dietary supplements.
Table 2. Mineral Composition of Rambutan Peel and Ellagitannin Rich Extract.
| element | rambutan peel (mg/kg) | ellagitannin rich extract (mg/kg) |
|---|---|---|
| Ca | 4528.88 ± 36.23 | 110.19 ± 0.55 |
| Cr | <12.5a | <12.5a |
| Cu | <25a | <25a |
| Fe | <12.5a | <12.5a |
| K | 5251.03 ± 78.77 | 150.19 ± 1.35 |
| Mg | 1063.67 ± 3.19 | 43.62 ± 1.09 |
| Mn | 176.95 ± 0.53 | <12.5a |
| Na | 211.50 ± 2.75 | 96.35 ± 1.06 |
| Ni | <12.5a | <12.5a |
| Zn | <12.5a | <12.5a |
The concentration was calculated on the basis of 0.4000 g of sample weight.
3.3. Antioxidant Activities of Extracts
The antioxidant activities of rambutan peel extracts in terms of hydrogen-donating and electron transfer capacity were compared with standard references such as vitamin C and BHT (Table 3). DPPH assay (hydrogen atoms or electrons donation ability) shows no significant difference between the crude extract, ellagitannin rich extract, and vitamin C, which exhibited strong antioxidant effect. However, there was high significant difference between the extracts vitamin E and BHT. The similarity in DPPH activity between crude extract and ellagitannin rich extract could be due to the presence of other bioactive compounds in the crude extract such as flavonoid,27,34 which has the ability to donate electrons or a hydrogen atom.56 ABTS assay, which investigates the antioxidant activity of chain-breaking and of hydrogen-donating antioxidants, shows significant difference between the extracts and the various standards, with vitamin C showing most effect of ABTS activity followed by ellagitannin rich and crude extracts. The reducing power assay shows no significant difference between vitamin C and ellagitannin rich extract with values of 15329.8 ± 1756.05 and 15088.76 ± 193.70 mmol of Fe(II)/kg of sample, respectively. Likewise, the crude extract and vitamin E show no significant differences in reducing power activity. Vitamin C is mostly used as a reference standard in the antioxidant test due to its consideration as the most important water-soluble antioxidant.57 The result revealed that the ellagitannin rich extract is as powerful as vitamin C with the ability to trap free radicals. The extract as a natural product is promising for antioxidant-based products preparation for human well-being.
Table 3. Antioxidant Activities of Crude and Ellagitannin Rich Extracts from Rambutan Peela.
| sample | DPPH IC50 (μg/mL) | ABTS IC50(mg/mL) | FRAP (mmol of Fe(II)/kg of sample) |
|---|---|---|---|
| vitamin C | 3.659 ± 0.128a | 0.126 ± 0.005a | 15329.8 ± 1756.05c |
| vitamin E | 12.353 ± 0.219b | 0.782 ± 0.017e | 4337.06 ± 223.81ab |
| BHT | 75.398 ± 1.446c | 0.445 ± 0.016d | 2348.24 ± 406.38a |
| crude extract | 6.369 ± 0.452a | 0.367 ± 0.021c | 5980.35 ± 256.27b |
| ellagitannin rich extract | 4.157 ± 0.226a | 0.201 ± 0.016b | 15088.76 ± 193.70c |
For values with the same letter, the difference is not statistically significant, while different letters are statistically significant (p ≤ 0.05).
3.4. Identification and Quantification of Polyphenolic Compounds
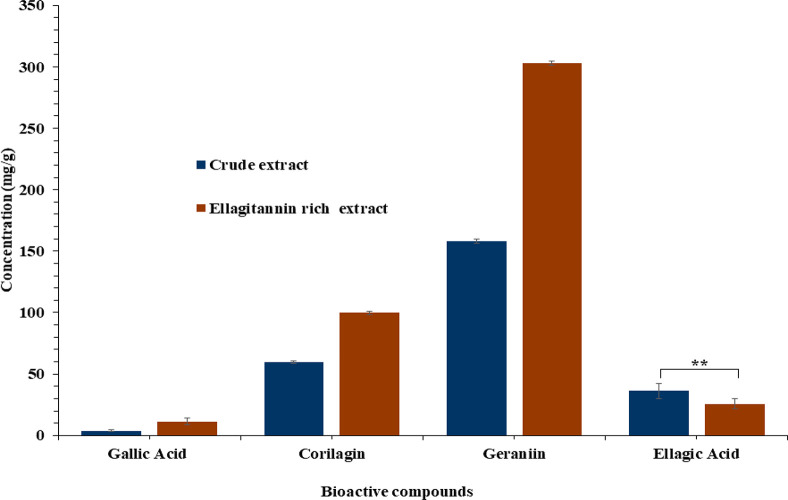
The main bioactive compounds in both extracts were identified and quantified on the basis of their chromatographic profiles and retention times at 280 nm. To determine the impact of partitioning on the compound presence in the extracts. The peaks of the main compounds gallic acid, geraniin, corilagin, and ellagic acid were identified at retention times of 6.074, 7.093, 7.368, and 8.798 min, respectively, on the basis of standard calibration curve (Supporting Information Figure S1). The HPLC chromatograms of the major compounds identified in the extracts are presented in Figure S2. The result (Figure 1) shows a highly significant difference between crude and ellagitannin rich extracts in terms of gallic acid, corilagin, and geraniin concentration. However, no significant difference was observed in relation to ellagic acid concentration in both extracts. The partitioning resulted in 66.2% increase in gallic acid, 40.4% increase in corilagin, and 47.8% increase in geraniin concentration. This could be explained by the removal of nonphenol components such as extractives and carbohydrate in the water fraction, leaving the active phenolic fraction.58,59 The nonphenol fractions in the crude extract could possibly form complexes with the phenolic active compounds, thus interfering with the biological activities of the extract. This was evident in the antioxidant activities in Table 3.
Figure 1.
Identification and quantification of phenolic compounds of crude and ellagitannin rich extracts from rambutan peel. Values with ** difference are not statistically significant (p ≤ 0.05).
The compounds were separated on the basis of their polarity and differential migration through the silica column at different rates. A total of 44 fractions were collected (Figure S3), identified, and categorized as gallic acid, geraniin, corilagin, and ellagic acid. The results revealed geraniin as the dominant fraction in the ellagitannin rich extract. This confirmed the result in Figure 1. Geraniin and its metabolites have been reported to possess a range of bioactive properties including antiviral activities against several types of viruses.39 The pharmacological and economic significance of this compound necessitated its exploration and separation. The basic monomer is hexahydroxydiphenol (HHDP), which equilibrated between the six-membered hemiacetal form and the five-membered hemiacetal form of the geraniin moiety, making it complicated. These couple with the structural resemblance in ellagitannins, especially geraniin and corilagin, making it difficult to separate them. However, the quick column approach looks promising for separation of geraniin with high activities. The fractions rich with geraniin were selected after HPLC analysis (Figure S4). The first fraction is enriched with geraniin of 166.4 mg/g followed by gallic acid (79.2 mg/g), while the second fraction is as much as 300.8 mg/g geraniin followed by corilagin (72.3 mg/g). It is evident that, in the fraction of high geraniin concentration, corilagin content also increases, while gallic acid decreased (7.1 mg/g). This may affirm the earlier accession of structural similarity in geraniin and corilagin that makes them difficult to isolate. The antioxidant contribution of the major ellagitannins was further established in the study.
3.5. Antioxidant Contribution of Extracts
Table 4 shows the DPPH free radical scavenging activity of the fractionated extracts with respect to the percentage contribution of the main ellagitannins. Two fractions rich with geraniin from the quick column chromatogram were selected to determine the antioxidant contributions of the major ellagitannins. Purified commercial compounds were used as the reference standard; however, all of the standard compounds showed antioxidant activity higher than Trolox except corilagin. The standard gallic acid showed radical scavenging activity, which is three times higher than that of Trolox. These may be as a result of their high efficiency in donating protons and electrons transfer ability. The rambutan peel extract rich in geraniin showed high capability of replacing Trolox as an antioxidant compound. The crude extract showed lower activity compared to Trolox; however, further fractionation to obtain ellagitannin rich and geraniin rich extract has significantly enhanced the antioxidant activity of the extracts. There was no significant difference between the standard geraniin and the geraniin rich fraction 2 (GRF2) from rambutan peel. Also, no significant difference was observed in ellagitannin rich, geraniin rich fraction 1 (GRF1), and standard ellagic acid. In all, only standard gallic acid showed activity higher than that of GRF2. The removal of nonbioactive components which form complexes and interfere with the biological activities of active ellagitannin through the fractionation process thus improved the antioxidant activity. Despite several research reports on antioxidant properties of rambutan peel extract, no attempt has been made to establish which compounds contributed the most in this regard. It is therefore established by this study that geraniin is not only the major ellagitannin in rambutan peel but also the major contributor to free radical scavenging activity. It is evident that there are other compounds other than gallic acid, geraniin, corilagin, and ellagic acid which have radical scavenging activity in the crude extract. The contribution of geraniin increased from 17.29% in crude extract to 61.34% in the GRF2. The contribution of corilagin increases with increase in geraniin contribution from 6.46% in crude extract to 14.74% in GRF2. Geraniin and corilagin belong to ellagitannin and were reported as the major compounds in rambutan peel.27 They are structurally identical and composed of hexahydroxydiphenolic acid linked by ester linkages to glucose molecules with high biochemical actions, including the ability to transfer electron and donate proton.60 The structural similarities of these compounds make it difficult to separate and give proportional yields of both compounds.
Table 4. DPPH Radical Scavenging Activity Value for Each Fraction and Contribution of Geraniina.
| DPPH
radical scavenging activity |
|||||
|---|---|---|---|---|---|
| ratio
of contribution (%)b |
|||||
| compoundc | TE2d (g/g) | gallic acid | geraniin | corilagin | ellagic acid |
| gallic acid (std) | 3.82 ± 0.08d | – | – | – | – |
| geraniin (std) | 1.58 ± 0.01c | – | – | – | – |
| corilagin (std) | 0.89 ± 0.07a | – | – | – | – |
| ellagic acid (std) | 1.43 ± 0.09bc | – | – | – | – |
| crude extract | 0.84 ± 0.03a | 0.42 | 17.29 | 6.46 | 3.98 |
| ellagitannin rich | 1.28 ± 0.08b | 18.69 | 50.54 | 16.65 | 4.31 |
| GRF1 | 1.27 ± 0.02b | 13.09 | 27.49 | 2.69 | 6.81 |
| GRF2 | 1.56 ± 0.11c | 1.45 | 61.34 | 14.74 | 5.88 |
For values with the same letter, the difference is not statistically significant, while different letters are statistically significant (p ≤ 0.05).
– = not measured.
std = standard.
TE2 = Trolox equivalent.
3.6. MTT Cytotoxicity Assay
The suitability of the extract for biological application in living tissue in terms of cytocompatibility is very critical. The metabolic viability of mouse fibroblast cells with the extract was evaluated using MTT cytotoxicity assay, on the basis of ISO 10993-5 standard. The OD values obtained (absorbance at 570 nm) after incubation show the extracts are nontoxic to the cells with cell viability above 70% (Figure 2). As predictable, the positive control (0.1% zinc diethyldithiocarbamate (ZDEC):RM-A) shows no viable cells, while the Thermanox (Nunc) coverslip as negative control shows 99% viable cells. The crude extract on the other hand had 93% cell viability which further decreased after partitioned to 74% at a concentration of 0.1 mg/mL. It is discovered that the extracts at a concentration of 0.1 mg/mL could be applied to living tissue without any toxic effect on the cells. This is in agreement with previous study which showed rambutan extract to be nontoxic to human dermal fibroblasts (HDFs).61
Figure 2.
Percentage of cell viability of rambutan peel extracts using MTT cytotoxicity assay.
3.7. Tyrosinase Inhibition Activity
The ability of the extract to inhibit tyrosinase, a key enzyme of melanogenesis, was studied. The extracts were compared with a standard tyrosinase inhibitor, kojic acid, and the result (Table 5) shows a highly significant difference between the extracts and kojic acid. There was no significant difference between crude and ellagitannin rich extracts. This means the partitioning process does not affect the phytoconstituents that chelate the copper ions at the active site of mushroom tyrosinase enzymes. Tyrosinase plays an important role in melanin synthesis, and oversynthesis of melanin leads to hyperpigmentation and other skin-related disorders. Therefore, a material with inhibitory activity of this enzyme is highly recommended for controlling melanin synthesis to have a whitening effect and prevent melanogenesis through the reduction of reactive oxygen species (ROS) levels in epidermal melanocytes.62−64 The current skin-whitening agent such as hydroquinone (benzene-1,4-diol or quinol), which is a derivative of benzene, is characterized by high toxicity;21 therefore, the moderate tyrosinase inhibitory activity of the extract makes it a potential candidate to be considered in skin-related application.
Table 5. Tyrosinase Activity of Crude and Ellagitannin Rich Rambutan Peel Extracta.
| sample | IC50 inhibitory concn (mg/mL) |
|---|---|
| kojic acid | 0.17 ± 0.02a |
| crude extract | 49.74 ± 3.40b |
| ellagitannin rich extract | 45.50 ± 1.99b |
For values with the same letter, the difference is not statistically significant, while different letters are statistically significant (p ≤ 0.05).
3.8. In Vitro Antimicrobial Activity of Extract
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of rambutan peel extracts were analyzed using aerobic S. aureus and S. epidermidis strains and an aerotolerant anaerobe C. acnes strain. MIC results (Table 6) show no differences in inhibition concentration of crude and ellagitannin rich extracts against S. aureus and S. epidermidis at 1.6 mg/mL and 800 μg/mL, respectively. However, that was not the case against anaerobic strain C. acnes, which is more sensitive to the ellagitannin rich extract at a lower concentration (800 μg/mL) than the crude extract (1600 μg/mL). The efficacy of the ellagitannin rich extract was evident in the bactericidal activity against all tested strains. The partitioning of the extract has maximized its efficacy by reducing the concentration required for MBC by 50%, relative to that of crude extract. The MBC value for the ellagitannin rich extract is better than those reported in previous studies.49 The antibacterial activity can be attributed to the presence of hexahydroxydiphenoyl moieties.7 They are reported to exert different mechanisms of action on microorganisms, including direct interference with microbial metabolism, adhesion capacity, enzymatic inhibition, removal of essential substances, metal ions complexations, and destruction of membrane cells.6 Even though the ellagitannin rich extract showed lower MIC than clindamycin against S. epidermidis and C. acnes, the two standard antibiotics (erythromycin and clindamycin) are highly effective against all of the bacterial stains at lower concentrations, probably due to their high level of purity. Nonetheless, further processing such as partitioning of the extract enhances its biological properties. The tested strains are major pathogens associated with human skin diseases. Thus, the efficacy of the ellagitannin rich extract against these pathogens made it a good natural product for skin-related formulations.
Table 6. Minimum Inhibitory and Bactericidal Concentrations of Rambutan Peel Extracts.
|
S. aureus |
S.
epidermidis |
C.
acnes |
||||
|---|---|---|---|---|---|---|
| compound (μg/mL) | MIC | MBC | MIC | MBC | MIC | MBC |
| clindamycin | 1280 | 1280 | 1280 | 1280 | 2.44 | 2.44 |
| erythromycin | 40 | 40 | 20 | 20 | 1.22 | 1.22 |
| crude extract | 1600 | 12800 | 800 | 12800 | 1600 | 6400 |
| ellagitannin rich | 1600 | 6400 | 800 | 6400 | 800 | 3200 |
4. Conclusion
The study revealed high amounts of beneficial mineral elements such as K, Ca, and Mg with other equally important ones (Cr, Cu, Fe, Mn, Na, Ni, and Zn) in low concentrations in the rambutan peel and extract. A high percentage of crude extract was recorded in the study using the Soxhlet apparatus. The partitioning of the extract resulted in significant increase in TPC, active compounds, and antioxidant activities, which was similar to that of commercial vitamin C serving as a standard. Quick column fractionation of ellagitannin rich extracts yielded fractions rich in geraniin with free radical scavenging activity similar to the commercial one. The ellagitannin rich extract was also highly effective against S. aureus, S. epidermidis, and C. acnes strains. It can be said that partitioning and further fractionation of extract is an effective way of improving its biological properties. Considering the valuable compounds and high biological activities discovered in the study, rambutan peel can be considered as a renewable natural material for bioactive compounds and mineral elements by pharmaceutical, cosmetic, and nutraceutical industries, for health and wellness products.
Acknowledgments
This research is supported by Kasetsart University Research and Development Institute, Grant No. FF(KU) 25.64, and the Office of the Ministry of Higher Education, Science, Research and Innovation, Thailand Science Research and Innovation through the Kasetsart University Reinventing University Program 2021. We are grateful to the Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University for providing facilities and funding under “Scholarships for International Graduate Students 2019”. We also acknowledge the staff of the Department of Herb and Bioactive Compound Technology Laboratory at Kasetsart Agricultural and Agro-Industrial Product Improvement Institute (KAPI) for their roles in the extraction and various analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04646.
Quick column fractionation, standard calibration curves, and HPLC chromatogram of extracts (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Krause J.; Tobin G.. Discovery, development, and regulation of natural products. In Using old solutions to new problems—Natural drug discovery in the 21st century; IntechOpen, 2013; Vol. 1, pp 1–35. 10.5772/56424. [DOI] [Google Scholar]
- Thitilertdecha N.; Rakariyatham N. Phenolic content and free radical scavenging activities in rambutan during fruit maturation. Sci. Hortic. 2011, 129 (2), 247–252. 10.1016/j.scienta.2011.03.041. [DOI] [Google Scholar]
- Sharma P.; Guleria P.; Kumar V.. Green nanotechnology for bioactive compounds delivery. In Biotechnological Production of Bioactive Compounds; Verma M. L., Chandel A. K., Eds.; Elsevier, 2020; Chapter 13, pp 391–407. 10.1016/B978-0-444-64323-0.00013-8. [DOI] [Google Scholar]
- Lautié E.; Russo O.; Ducrot P.; Boutin J. A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front Pharmacol. 2020, 11, 307. 10.3389/fphar.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F.; Passon M.. Characterization and Quantification of Polyphenols in Fruits. In Polyphenols in Plants, 2nd ed.; Watson R. R., Ed.; Academic Press, 2019; Chapter 7, pp 111–121. 10.1016/B978-0-12-813768-0.00007-4. [DOI] [Google Scholar]
- Faggian M.; Bernabè G.; Ferrari S.; Francescato S.; Baratto G.; Castagliuolo I.; Dall’Acqua S.; Peron G. Polyphenol-Rich Larix decidua Bark Extract with Antimicrobial Activity against Respiratory-Tract Pathogens: A Novel Bioactive Ingredient with Potential Pharmaceutical and Nutraceutical Applications. Antibiotics (Basel) 2021, 10 (7), 789. 10.3390/antibiotics10070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekambaram S. P.; Perumal S. S.; Balakrishnan A. Scope of Hydrolysable Tannins as Possible Antimicrobial Agent. Phytother. Res. 2016, 30 (7), 1035–1045. 10.1002/ptr.5616. [DOI] [PubMed] [Google Scholar]
- Funatogawa K.; Hayashi S.; Shimomura H.; Yoshida T.; Hatano T.; Ito H.; Hirai Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol. Immunol. 2004, 48 (4), 251–61. 10.1111/j.1348-0421.2004.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Puljula E.; Walton G.; Woodward M. J.; Karonen M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25 (16), 3714. 10.3390/molecules25163714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeriglio A.; Barreca D.; Bellocco E.; Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174 (11), 1244–1262. 10.1111/bph.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T.; Ito H. Tannins of constant structure in medicinal and food plants—hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16 (3), 2191–2217. 10.3390/molecules16032191. [DOI] [Google Scholar]
- Liu C.; Liu H.; Huang H.; Hao J.; Lv Y.; Zhang J.; Ma Y.; Wu C.; Qin R.; Yang X. Corilagin induces laryngeal cancer antiproliferation and inhibits growth factor and cytokine signaling pathways in vitro and in vivo. J. Funct. Foods 2020, 69, 103947. 10.1016/j.jff.2020.103947. [DOI] [Google Scholar]
- Dias R.; Perez-Gregorio M. R.; Mateus N.; De Freitas V. Interaction study between wheat-derived peptides and procyanidin B3 by mass spectrometry. Food Chem. 2016, 194, 1304–1312. 10.1016/j.foodchem.2015.08.108. [DOI] [PubMed] [Google Scholar]
- Molino S.; Casanova N. A.; Rufián Henares J. Á.; Fernandez Miyakawa M. E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68 (10), 2836–2848. 10.1021/acs.jafc.9b00590. [DOI] [PubMed] [Google Scholar]
- Berdowska I.; Matusiewicz M.; Fecka I. Punicalagin in Cancer Prevention—Via Signaling Pathways Targeting. Nutrients 2021, 13 (8), 2733. 10.3390/nu13082733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang S. C. W.; Palanisamy U. D.; Kadir K. A. Effects of geraniin (rambutan rind extract) on blood pressure and metabolic parameters in rats fed high-fat diet. J. Integr. Med. 2019, 17 (2), 100–106. 10.1016/j.joim.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Thitilertdecha N.; Chaiwut P.; Saewan N. In vitro antioxidant potential of Nephelium lappaceum L. rind extracts and geraniin on human epidermal keratinocytes. Biocatal. Agric. Biotechnol. 2020, 23, 101482. 10.1016/j.bcab.2019.101482. [DOI] [Google Scholar]
- Rad H. H.; Yamashita T.; Jin H.-Y.; Hirosaki K.; Wakamatsu K.; Ito S.; Jimbow K. Tyrosinase-related proteins suppress tyrosinase-mediated cell death of melanocytes and melanoma cells. Exp. Cell Res. 2004, 298 (2), 317–328. 10.1016/j.yexcr.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Qian W.; Liu W.; Zhu D.; Cao Y.; Tang A.; Gong G.; Su H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20 (1), 173–185. 10.3892/etm.2020.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.-Y.; Kim J.. Synthesis and Biological Evaluation of the Anti-Melanogenesis Effect of Coumaric and Caffeic Acid-Conjugated Peptides in Human Melanocytes. Front. Pharmacol. 2020, 11.922. 10.3389/fphar.2020.00922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira R. d. S.; Rocha P. R.; Polonini H. C.; Brandão M. A. F.; Chaves M. d. G. A. M.; Raposo N. R. B. Mushroom tyrosinase inhibitory activity and major fatty acid constituents of Amazonian native flora oils. Braz. J. Pharm. Sci. 2012, 48 (3), 399–404. 10.1590/S1984-82502012000300006. [DOI] [Google Scholar]
- Kang S. J.; Choi B. R.; Lee E. K.; Kim S. H.; Yi H. Y.; Park H. R.; Song C. H.; Lee Y. J.; Ku S. K. Inhibitory Effect of Dried Pomegranate Concentration Powder on Melanogenesis in B16F10 Melanoma Cells; Involvement of p38 and PKA Signaling Pathways. Int. J. Mol. Sci. 2015, 16 (10), 24219–24242. 10.3390/ijms161024219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín J. C.; García-Conesa M. T.; Tomás-Barberán F. A. Nutraceuticals: facts and fiction. Phytochemistry 2007, 68 (22–24), 2986–3008. 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Munekata P. E. S.; Pateiro M.; Barba F. J.; Dominguéz R.; Gagaoua M.; Lorenzo J. M.. Development of new food and pharmaceutical products: Nutraceuticals and food additives. In Advances in Food and Nutrition Research; Lorenzo J. M., Barba F. J., Eds.; Academic Press, 2020; Vol. 92, Chapter 3, pp 53–96. 10.1016/bs.afnr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- AlAli M.; Alqubaisy M.; Aljaafari M. N.; AlAli A. O.; Baqais L.; Molouki A.; Abushelaibi A.; Lai K.-S.; Lim S.-H. E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26 (9), 2540. 10.3390/molecules26092540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycia K.; Ivanišová E. Physicochemical and antioxidant properties of wheat bread enriched with hazelnuts and walnuts. Foods 2020, 9 (8), 1081. 10.3390/foods9081081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgbo S.; Sukatta U.; Kamonpatana P.; Sukyai P. Ohmic heating extraction and characterization of rambutan (Nephelium lappaceum L.) peel extract with enhanced antioxidant and antifungal activity as a bioactive and functional ingredient in white bread preparation. Food Chem. 2022, 382, 132332. 10.1016/j.foodchem.2022.132332. [DOI] [PubMed] [Google Scholar]
- Chai K. F.; Adzahan N. M.; Karim R.; Rukayadi Y.; Ghazali H. M. Selected physicochemical properties of registered clones and wild types rambutan (Nephelium lappaceum L.) fruits and their potentials in food products. Sains Malays. 2018, 47 (7), 1483–1490. 10.17576/jsm-2018-4707-16. [DOI] [Google Scholar]
- Chai K. F.; Chang L. S.; Adzahan N. M.; Karim R.; Rukayadi Y.; Ghazali H. M. Physicochemical properties and toxicity of cocoa powder-like product from roasted seeds of fermented rambutan (Nephelium lappaceum L.) fruit. Food Chem. 2019, 271, 298–308. 10.1016/j.foodchem.2018.07.155. [DOI] [PubMed] [Google Scholar]
- Arenas M. G. H.; Angel D. N.; Damian M. T. M.; Ortiz D. T.; Díaz C. N.; Martinez N. B. Characterization of rambutan (Nephelium lappaceum) fruits from outstanding mexican selections. Rev. Bras. Frutic. 2010, 32, 1098–1104. 10.1590/S0100-29452011005000004. [DOI] [Google Scholar]
- Hernández-Hernández C.; Aguilar C. N.; Rodríguez-Herrera R.; Flores-Gallegos A. C.; Morlett-Chávez J.; Govea-Salas M.; Ascacio-Valdés J. A. Rambutan(Nephelium lappaceum L.):Nutritional and functional properties. Trends. Food Sci. Technol. 2019, 85, 201–210. 10.1016/j.tifs.2019.01.018. [DOI] [Google Scholar]
- Rakariyatham K.; Zhou D.; Rakariyatham N.; Shahidi F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenolic compounds and use as functional ingredients in food and health applications. J. Funct. Foods 2020, 67, 103846. 10.1016/j.jff.2020.103846. [DOI] [Google Scholar]
- Yahya N. A.; Attan N.; Wahab R. A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018, 112, 69–85. 10.1016/j.fbp.2018.09.002. [DOI] [Google Scholar]
- Monrroy M.; Araúz O.; García J. R. Active compound identification in extracts of N. lappaceum peel and evaluation of antioxidant capacity. J. Chem. 2020, 2020, 4301891. 10.1155/2020/4301891. [DOI] [Google Scholar]
- Cheng H. S.; Ton S. H.; Abdul Kadir K. Ellagitannin geraniin: a review of the natural sources, biosynthesis, pharmacokinetics and biological effects. Phytochem. Rev. 2017, 16 (1), 159–193. 10.1007/s11101-016-9464-2. [DOI] [Google Scholar]
- Elendran S.; Muniyandy S.; Lee W. W.; Palanisamy U. D. Permeability of the ellagitannin geraniin and its metabolites in a human colon adenocarcinoma Caco-2 cell culture model. Food Funct. 2019, 10 (2), 602–615. 10.1039/C8FO01927D. [DOI] [PubMed] [Google Scholar]
- Graça V. C.; Ferreira I. C. F. R.; Santos P. F. Phytochemical composition and biological activities of Geranium robertianum L.: A review. Ind. Crops Prod. 2016, 87, 363–378. 10.1016/j.indcrop.2016.04.058. [DOI] [Google Scholar]
- Elendran S.; Wang L. W.; Prankerd R.; Palanisamy U. D. The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm. Biol. 2015, 53 (12), 1719–1726. 10.3109/13880209.2014.1003356. [DOI] [PubMed] [Google Scholar]
- Abdul Ahmad S. A.; Palanisamy U. D.; Tejo B. A.; Chew M. F.; Tham H. W.; Syed Hassan S. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol. J. 2017, 14 (1), 229. 10.1186/s12985-017-0895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu F.; Mat Taib C. N.; Mohd Moklas M. A.; Mohd Akhir S. Antioxidant properties of crude extract, partition extract, and fermented medium of dendrobium sabin flower. Evid. Based Complement. Alternat. Med. 2017, 2017, 2907219. 10.1155/2017/2907219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K.; Wu X.; Liu R. H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51 (3), 609–614. 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Senapitakkul V.; Vanitjinda G.; Torgbo S.; Pinmanee P.; Nimchua T.; Rungthaworn P.; Sukatta U.; Sukyai P. Pretreatment of Cellulose from Sugarcane Bagasse with Xylanase for Improving Dyeability with Natural Dyes. ACS Omega 2020, 5 (43), 28168–28177. 10.1021/acsomega.0c03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwandter H. Universal 5-min on-line method for extracting and isolating pesticide residues and industrial chemicals. Fresenius Z. Anal. Chem. 1985, 322 (8), 752–754. 10.1007/BF00489393. [DOI] [Google Scholar]
- Sukatta U.; Rugthaworn P.; Seangyen W.; Tantaterdtam R.; Smitthipong W.; Chollakup R. Prospects for rambutan peel extract as natural antioxidant on the aging properties of vulcanized natural rubber. SPE Polym. 2021, 2 (3), 199–209. 10.1002/pls2.10042. [DOI] [Google Scholar]
- Torgbo S.; Sukyai P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. 10.1016/j.matchemphys.2019.121868. [DOI] [Google Scholar]
- Kubo I.; Kinst-Hori I.; Chaudhuri S. K.; Kubo Y.; Sánchez Y.; Ogura T. Flavonols from Heterotheca inuloides: Tyrosinase Inhibitory Activity and Structural Criteria. Bioorg. Med. Chem. 2000, 8 (7), 1749–1755. 10.1016/S0968-0896(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Saewan N.; Koysomboon S.; Chantrapromma K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J. Med. Plants Res. 2011, 5 (6), 1018–1025. [Google Scholar]
- Sunthornvarabhas J.; Rungthaworn P.; Sukatta U.; Juntratip N.; Sriroth K. Antimicrobial Tendency of Bagasse Lignin Extracts by Raman Peak Intensity. Sugar Tech 2020, 22 (4), 697–705. 10.1007/s12355-019-00778-x. [DOI] [Google Scholar]
- Sukatta U.; Rugthaworn P.; Khanoonkon N.; Anongjanya P.; Kongsin K.; Sukyai P.; Harnkarnsujarit N.; Sothornvit R.; Chollakup R. Rambutan (Nephelium lappaceum) peel extract: Antimicrobial and antioxidant activities and its application as a bioactive compound in whey protein isolate film. Songklanakarin J. Sci. Technol. 2021, 43 (1), 37–44. [Google Scholar]
- Thitilertdecha N.; Teerawutgulrag A.; Rakariyatham N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT - Food Sci. Technol. 2008, 41 (10), 2029–2035. 10.1016/j.lwt.2008.01.017. [DOI] [Google Scholar]
- Palanisamy U.; Manaharan T.; Teng L. L.; Radhakrishnan A. K. C.; Subramaniam T.; Masilamani T. Rambutan rind in the management of hyperglycemia. Food Res. Inter. 2011, 44 (7), 2278–2282. 10.1016/j.foodres.2011.01.048. [DOI] [Google Scholar]
- Elbagermi M. A.; Edwards H. G. M.; Alajtal A. I. Monitoring of Heavy Metal Content in Fruits and Vegetables Collected from Production and Market Sites in the Misurata Area of Libya. ISRN Anal. Chem. 2012, 2012, 827645. 10.5402/2012/827645. [DOI] [Google Scholar]
- Meepun N.; Saguansakbaramee N.; Wongchuphan R. Analysis of lead and cadmium contents in local vegetables in Surat Thani, Thailand. Walailak J. Sci. Technol. 2014, 11 (6), 455–461. [Google Scholar]
- Alimentarius C.General standard for contaminants and toxins in food and feed (Codex STAN 193-1995). Dokipedia, 2015; http://dokipedia.ru/document/5197124 (Accessed on July 10, 2022).
- Joint F.; Commission F. W. C. A. Codex Committee on Food Addititves and Contaminants . Report of the 34th Session of the Codex Committee on Food Additives and Contaminants, Rotterdam, The Netherlands, 11–15 March 2002; Food and Agriculture Organization of the United Nations (FAO), 2002; https://agris.fao.org/agris-search/search.do?recordID=XF2016079853 (Accessed on July 10, 2022).
- Farooq U.; Shafi A.; Akram K.; Hayat Z.. Fruits and nutritional security. In Fruit Crops; Srivastava A. K., Hu C., Eds.; Elsevier, 2020; Chapter 1, pp 1–12. 10.1016/B978-0-12-818732-6.00001-0. [DOI] [Google Scholar]
- Ekin S.; Bayramoglu M.; Goktasoglu A.; Ozgokce F.; Kiziltas H. Antioxidant activity of aqueous and ethanol extracts of Crataegus meyeri Pojark leaves and contents of vitamin, trace element. J. Chil. Chem. Soc. 2017, 62 (4), 3661–3667. 10.4067/s0717-97072017000403661. [DOI] [Google Scholar]
- Do Q. D.; Angkawijaya A. E.; Tran-Nguyen P. L.; Huynh L. H.; Soetaredjo F. E.; Ismadji S.; Ju Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug. Anal. 2014, 22 (3), 296–302. 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.-W.; Lin L.-G.; Ye W.-C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018, 13, 20. 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimse S. B.; Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015, 5 (35), 27986–28006. 10.1039/C4RA13315C. [DOI] [Google Scholar]
- Lee Y. R.; Cho H. M.; Park E. J.; Zhang M.; Doan T. P.; Lee B. W.; Cho K. A.; Oh W. K. Metabolite Profiling of Rambutan (Nephelium lappaceum L.) Seeds Using UPLC-qTOF-MS/MS and Senomorphic Effects in Aged Human Dermal Fibroblasts. Nutrients 2020, 12 (5), 1430. 10.3390/nu12051430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer A.; Chisvert A.; Salvador A. Environmentally friendly LC for the simultaneous determination of ascorbic acid and its derivatives in skin-whitening cosmetics. J. Sep. Sci. 2008, 31 (2), 229–236. 10.1002/jssc.200700414. [DOI] [PubMed] [Google Scholar]
- Park K. M.; Kwon K. M.; Lee S. H. Evaluation of the Antioxidant Activities and Tyrosinase Inhibitory Property from Mycelium Culture Extracts. Evid. Based Complement. Alternat. Med. 2015, 2015, 616298. 10.1155/2015/616298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denat L.; Kadekaro A. L.; Marrot L.; Leachman S. A.; Abdel-Malek Z. A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Invest. Dermatol. 2014, 134 (6), 1512–1518. 10.1038/jid.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.