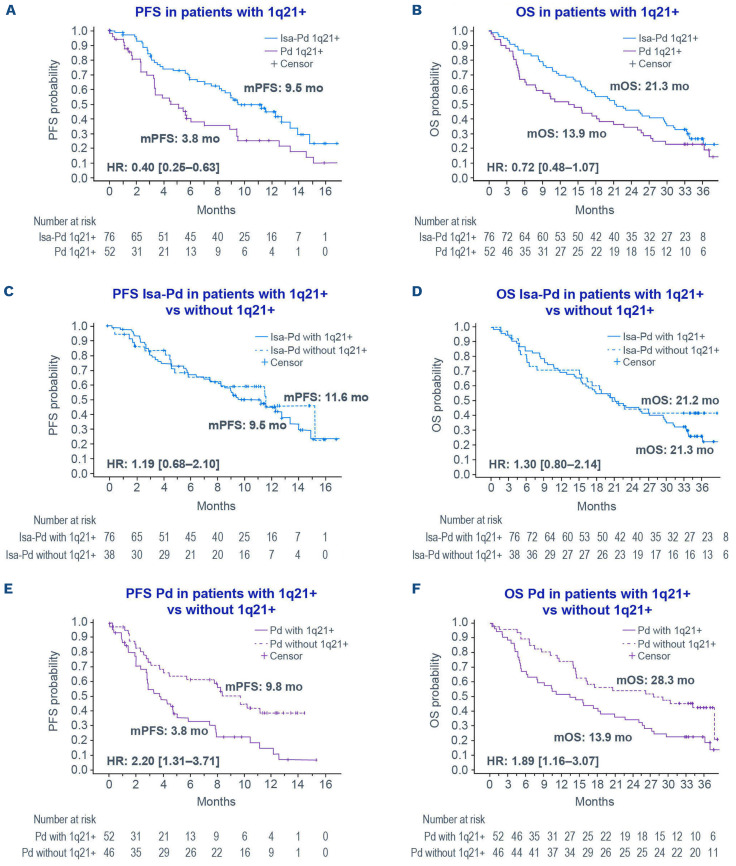
Figure 1.
Survival outcomes in patients with relapsed/refractory multiple myeloma in the ICARIA-MM study according to treatment received and 1q21+ status. (A-F) Kaplan-Meier estimates of progression-free survival and overall survival from the ICARIA-MM study in the subgroup of patients with 1q21+ treated with isatuximab (Isa) plus pomalidomide–dexamethasone (Pd) versus Pd (A, B), Isa-Pd with 1q21+ versus Isa-Pd without 1q21+ (C, D), and Pd with 1q21+ versus Pd without 1q21+ (E, F). Progression-free survival was defined as the time from randomization to first documentation of progressive disease before initiation of anti-myeloma therapy or death from any cause, whichever came first. Progression-free survival data were analyzed as per the ICARIA-MM primary analysis cutoff date (October 11, 2018). Overall survival data were analyzed at the second interim cutoff date (October 1, 2020). Efficacy analyses were performed on the intention-to-treat population and summarized by assigned treatment. Confidence intervals are 95% for all Kaplan-Meier plots. 1q21+ definition: ≥3 copies, 30% cutoff, with or without high-risk chromosomal abnormalities. PFS: progression-free survival; mPFS: median progression-free survival; mo: months; HR: hazard ratio; OS: overall survival; mOS: median overall survival.