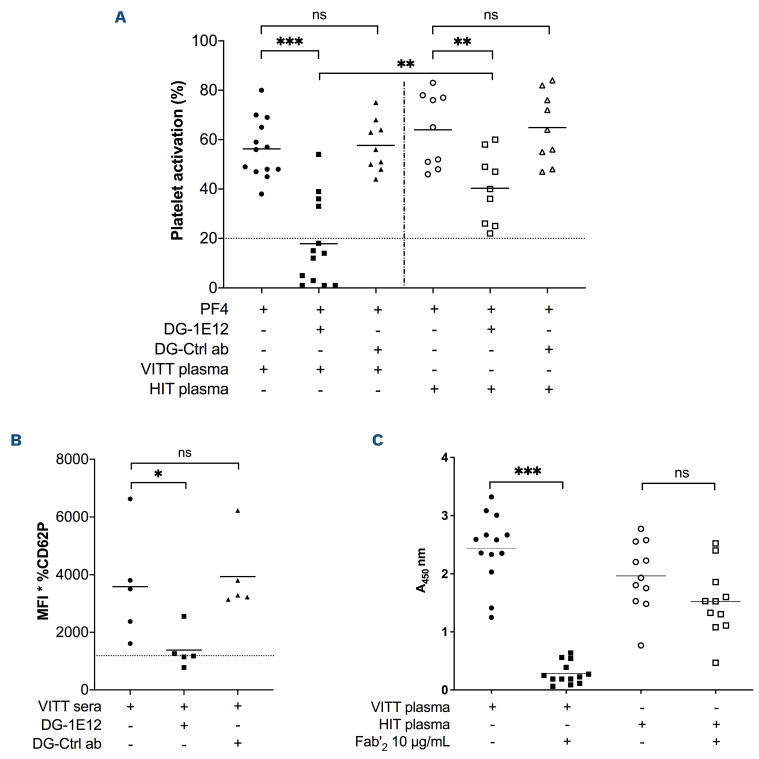
Figure 3.
Deglycosylated 1E12 inhibits platelet activation induced by human vaccine-induced immune thrombotic thrombocytopenia antibodies. (A) Platelet activation induced by vaccine-induced immune thrombotic thrombocytopenia (VITT) (n=13) and HIT plasma samples (n=9) in PF4-modified serotonin release assay (PF4-SRA) with or without DG-1E12 or DG-Ctrl antibody (ab) (50 μg/mL). The maximal serotonin release values that were measured are expressed in percentages of (%), with means (horizontal lines). Experiments using DG-Ctrl ab (deglycosylated form of control antibody) was performed with all available VITT plasma samples (n=9) and HIT plasmas (n=9). All SRA experiments were performed in the presence of human PF4 (10 μg/mL). Platelet activation induced by VITT samples (n=5) added in whole blood with or without DG-1E12 or DG-Ctrl antibody (100 μg/mL), and measured by flow cytometry (B). CD62P expression is shown as the mean fluorescence intensity (MFI) of the gated events multiplied by the percentage of gated events. Each data point represents the averaged result from 3 different tests with 1 sample. Horizontal lines indicate the mean values obtained with the five VITT samples. Effects of Fab’2 fragment of 1E12 (10 μg/mL) on VITT (n=13) or HIT (n=11) antibody binding to PF4/PVS evaluated by competitive enzyme immunoassays (C).