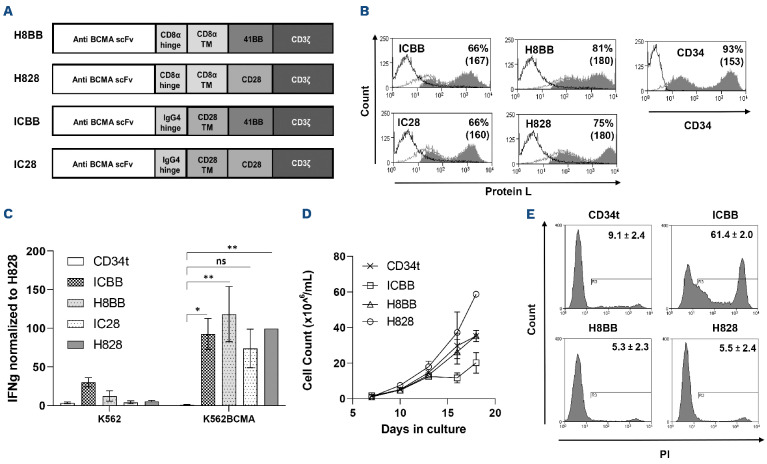
Figure 1.
Characterization of anti-BCMA based chimeras. (A) Schematic representation of the different designs of anti-BCMA chimeric receptors described in the Materials and Methods section. (B) Anti-CD3-activated human primary peripheral blood mononuclear cells (PBMC) were transduced with the different anti-BCMA chimeric antigen receptor (CAR) or with truncated CD34 (CD34t, control gene), as indicated. These cells were stained using Protein L or anti-CD34 antibody, respectively. Transgene expression was assessed by flow cytometry. The dotted line represents the staining of the mock-transduced control cells. The percentage of positive cells and the mean fluorescence intensity (MFI) (in brackets) are shown. The graph represents the average of 4 different donors and the difference in the staining between the population transduced with BCMA-CAR and the control population was found statistically significant (P<0.05; calculated using a Student’s paired t-test). (C) Co-culture of 1e5 transduced cells with 1e5 K562-BCMA or K562 cells (negative control) for 16 hours. Interferon γ (IFNγ) secreted in the co-culture supernatant was measured by enzyme-linked immunosorbant assay. These results are representative of 3 independent experiments. (D) Cell concentration was determined for BCMA-CAR-T cells and control T cells every 2-3 days for 3 weeks. These results are representative of 3 independent experiments with different donors (ICBB vs. H8BB/H828, P<0.05; by two-way ANOVA). (D) Cell viability was assessed 3 weeks following transduction. BCMA-CAR T cells were stained with propidium iodide (PI) and analyzed by flow cytometry, ICBB vs. H8BB/H828, P<0.001, by paired Student’s t-test. BCMA: B-cell maturation antigen.