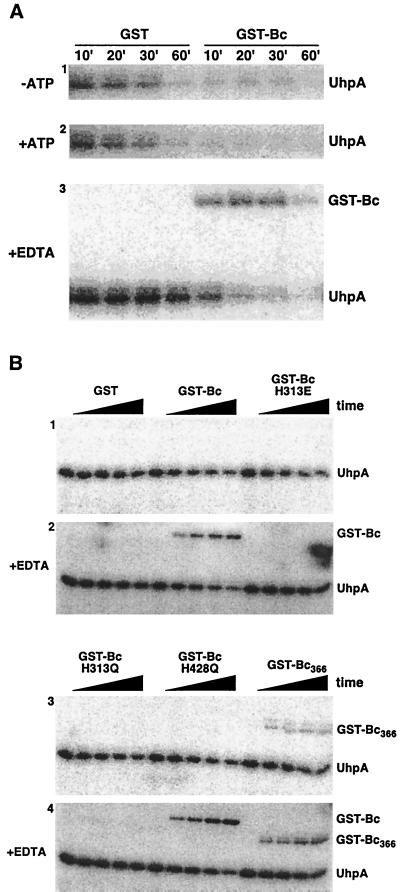
FIG. 3.
Dephosphorylation of P-UhpA by GST-Bc. (A) UhpA (15 μM) was phosphorylated by incubation with 20 mM acetyl [32P]phosphate as described in Materials and Methods. P-UhpA was isolated by adsorption to Ni2+-conjugated agarose beads, washed, and eluted to remove excess acetyl [32P]phosphate. P-UhpA dephosphorylation was measured following further incubation in the presence of 1.5 μM GST or GST-Bc in the absence of ATP (first panel), in the presence of 1 mM ATP (second panel), and in the presence of 15 mM EDTA (third panel). Samples were removed at the indicated times and separated by SDS-PAGE. The location of phosphorylated proteins was determined by PhosphorImager analysis. (B) Reverse phosphotransfer was detected by incubation of P-UhpA with GST-Bc variants carrying substitutions in the H box (H313E and H313Q) or the catalytic domain (H428Q) (49) or with the nucleotide-binding domain removed (GST-Bc366). Following incubation with P-UhpA, portions were removed at 0, 5, 10, 20, and 30 min and analyzed as described for panel A. Hydrolysis of P-UhpA was determined in the absence of 15 mM EDTA (panels 1 and 3) and in its presence (panels 2 and 4).