Figure 1.
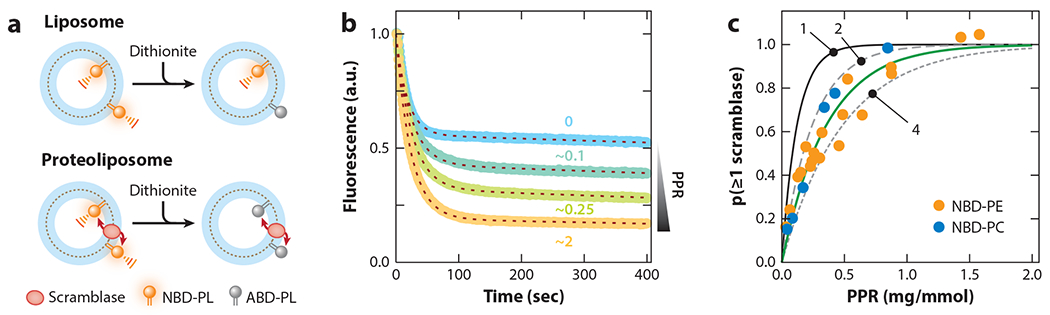
Phospholipid scrambling by opsin in reconstituted vesicles. (a) Scramblase assay Large unilamellar vesicles are reconstituted with a trace amount of fluorescent phospholipid [nitrobenzoxadiazole-phospholipid (NBD-PL)] and with (proteoliposome) or without (liposome) opsin. On adding dithionite to the vesicles, NBD-PL molecules in the outer leaflet are bleached to nonfluorescent aminobenzoxadiazole-phospholipid (ABD-PL). For liposomes (top), dithionite addition lowers fluorescence to approximately 50% of its initial value; for a proteoliposome with a functional scramblase (bottom), fluorescence is eliminated as NBD-PLs in the protected inner leaflet are scrambled to the dithionite-accessible outer leaflet. (b) Representative fluorescence traces corresponding to vesicles reconstituted at different protein–phospholipid ratios (PPRs). The blue trace (PPR = 0) corresponds to protein-free liposomes; the remaining traces correspond to vesicles with increasing PPR (approximately 0.1, 0.25, and 2 mg protein per mmol phospholipid). The dashed lines overlaying each trace represent data fits (mono-exponential decay with a half-time of approximately 15 s, plus a shallow linear component with slope approximately 10−4 s−1). (c) Protein-dependence data indicate that opsin reconstitutes as a dimer or tetramer. The solid, long-dashed, and short-dashed lines correspond to the result expected for the functional reconstitution of opsin as a monomer, dimer, or tetramer, respectively (indicated as 1, 2, and 4, respectively). The green line is the fit to the experimental data [filled circles correspond to assays done with NBD-phosphatidylethanolamine (PE) or NBD-phosphatidylcholine (PC)]. The data in panels b and c are from Goren et al. (32) and Ploier et al. (88).
