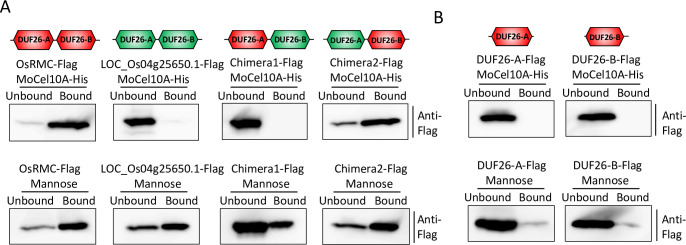
Fig 9. OsRMC DUF26-B determines its binding to CBM1.
(A) OsRMC, LOC_Os04g25650.1, and their chimeric proteins were produced in N. benthamiana and assayed for MoCel10A (CBM1: top) and mannose (bottom) binding. Simplified schemes of OsRMC (235 amino acids; red) and LOC_Os04g25650.1 (251 amino acids; green) as well as two newly generated chimeric proteins, Chimera1 (244 amino acids, comprising 97 aa [24–120] of OsRMC and 147 aa [135–281] of LOC_Os04g25650.1) and Chimera2 (242 amino acids, comprising 104 aa [31–134] of LOC_Os04g25650.1 and 138 aa [121–258] of OsRMC) are shown. Flag-tagged proteins were assayed for binding to MoCel10A-His and to mannose. Unbound and bound fractions were subjected to immunoblot analysis with an anti-Flag antibody. (B) Binding assay of single DUF26 domains (DUF26-A and DUF26-B) of OsRMC to MoCel10A (CBM1: top) and mannose (bottom).