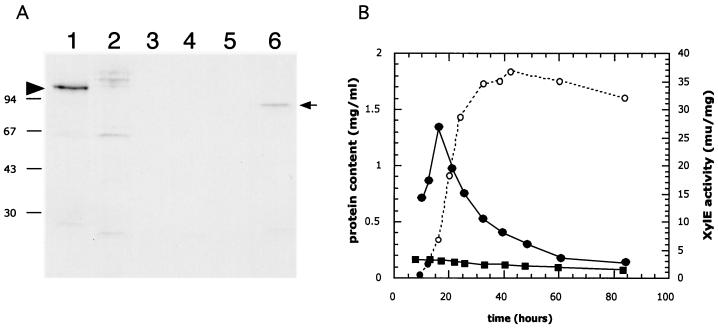
FIG. 5.
Developmental control of acoA. (A) Accumulation of AcoA in cultures grown on solid medium. AcoA was detected in extracts of surface-grown cultures by immunoblotting using antibodies against E. coli AcnA. MT1110/pKC1218 (lanes 1 through 5) and BZ4 (lane 6) were grown on R2YE (containing 100 μg of apramycin/ml) plates that had been covered with cellophane disks. Crude extracts prepared from samples harvested after 30 h (lane 1 and 6), 44 h (lane 2), 72 h (lane 3), 96 h (lane 4) and 120 h (lane 5) were resolved by SDS–10% PAGE and transferred to a PVDF nylon membrane. Immunodetection was performed as described in Materials and Methods. The left arrowhead indicates the position of the wild-type AcoA (MT1110/pKC1218), while the arrow on the right points to the presumed truncated or degraded AcoA detected in extracts of BZ4. The molecular masses of the protein marker are indicated on the left (in kilodaltons). (B) Activity of the acoA promoter in liquid cultures. S. coelicolor MT1110 strains carrying pIJ2839 (a XylE-based promoter probe vector) or a derivative (pVHP171) containing a 277-bp NsiI/XmnI fragment (Fig. 6A) extending from within the acoA ORF to 269 bp upstream were grown in YEME liquid medium. Crude extracts prepared from cultures throughout growth (open circles, protein content) were assayed for XylE specific activity (closed squares, pIJ2839; closed circles, pVHP171).