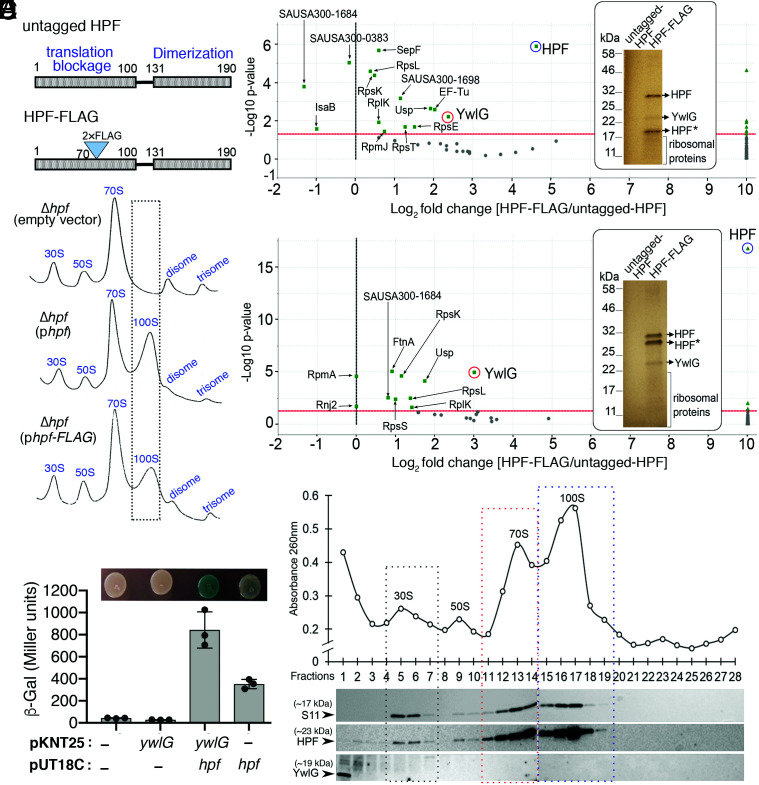
Fig. 1.
Identification of HPF interaction partners in S. aureus. (A) Schematic diagram showing the domain structures of S. aureus HPF and the position of the 2×FLAG in-frame fusion. The N-terminal domain (NTD) of HPF prevents translational initiation, whereas the carboxyl-terminal domain (CTD) promotes the dimerization of 70S ribosomes to form the 100S complex. (B) Ribosome sedimentation profiles of the Δhpf-null mutant bearing either empty vector, untagged HPF, or 2×FLAG-HPF. Five Abs260 units of RNA were loaded on a 5–30% sucrose gradient (x axes), and the levels of ribosomal complexes were monitored by the Abs260 (y axes). FLAG-tag insertion in HPF did not affect ribosome assembly and only modestly reduced the formation of 100S ribosomes. (C and D) Affinity copurification of HPF-binding proteins. TSB cultures of strains from A were harvested during the exponential (C) (4 h) or stationary phase (D) (18 h) at 37 °C. The total proteins were incubated with anti-FLAG M2 magnetic beads, and HPF and HPF-binding candidates were eluted as described in the Materials and Methods. Ten percent of the eluate was analyzed on an SDS-PAGE gel (Insets, images were pseudocolored), and protein bands that were unique in FLAG-tagged samples were identified by trypsin digestion followed by liquid chromatography (LC)-MS/MS. HPF* indicates degraded intermediates. The remaining eluate was directly subjected to gel-free quantitative MS, and differentially enriched proteins were visualized in Scaffold 4.11.0 and are presented in volcano plots (n = 2, P < 0.005, Fisher’s test with Benjamini–Hochberg parameters). (E) Validation of the HPF–YwlG interaction using a bacterial 2-hybrid (BACTH) system. T25-YwlG and T18-HPF chimeras are indicated on the x axis. High β-galactosidase activity is indicative of a direct YwlG–HPF interaction, consistent with chromogenic detection of LacZ accumulation on LB-X-Gal-isopropyl ß-D-1-thiogalactopyranoside solid agar after 24–30 h at 30 °C (Top). Each bar represents the average of three independent biological replicates (±SD). (F) YwlG does not associate with the mature ribosomes. Ribosome sedimentation profile of the wild-type JE2 strain grown in TSB to late exponential phase (optical density at 600 nm [OD600] = 1.6) at 37 °C. Five Abs260 units of RNA were ultracentrifuged through a 5–30% sucrose gradient (x axis), and the abundance of ribosomal complexes was monitored by Abs260 (y axis). Fractions were collected and probed with anti-HPF, anti-YwlG, and anti-S11 (30S subunit marker) on Western blots.