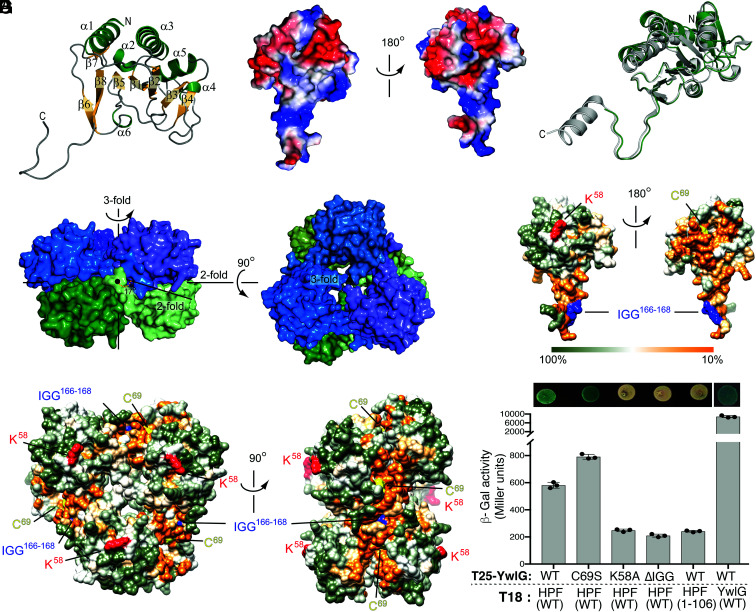
Fig. 6.
X-ray crystal structure of S. aureus YwlG and mutagenesis of YwlG and HPF. (A) Folding topology of YwlG shown on the monomer with secondary structural elements annotated. (B) Charges of amino acid side chains (red = negative; blue= positive) mapped onto the surface of a YwlG monomer. (C) Superposition of a YwlG monomer (green) with the homologous structure of the conserved hypothetical protein TT1679 from Thermus thermophilus (gray, PDB 1V8D). (D) Assembly of YwlG into a hexamer composed of two stacked trimeric rings. (E and F) Sequence conservation mapped onto the surface of the YwlG monomer (E) and hexamer (F), with K58, C69, and the 166IGG168 motif highlighted in red, yellow, and blue, respectively. (G) BACTH assay. HPF-CTD is important for HPF–YwlG interaction, whereas YwlG(K58A)-T25 and YwlG(ΔIGG)-T25 proteins are unstable (SI Appendix, Fig. S10), resulting in the lack of β-Gal activity. Interaction between YwlG-T25 and YwlG-18 independently validates the oligomerization status of YwlG in the crystals. The error bars indicate mean ± SD, n = 3.