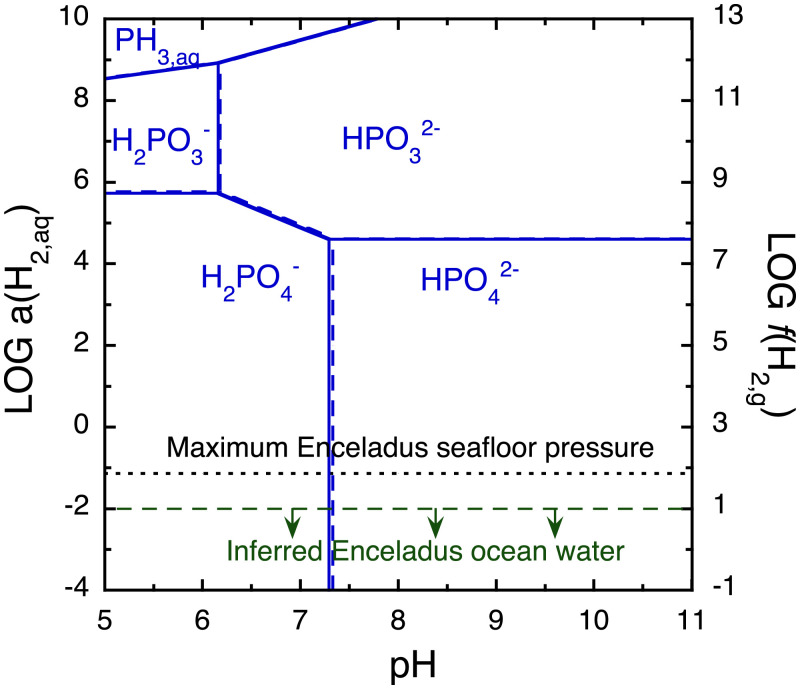
Fig. 1.
Thermodynamically favored form of dissolved phosphorus as a function of pH and equilibrium oxidation state (as activity of dissolved hydrogen, or fugacity of hydrogen gas in bars) at 0 °C and 70 bars (1 bar for reference in dashed lines). Within its predominance region, the indicated species would have the highest activity out of all aqueous P species if equilibrium is reached. The observationally based upper limit on a(H2,aq) (dashed green line) is from Waite et al. (4), and the theoretical upper limit on f(H2,g) (dotted black line) is from Glein et al. (21). Diphosphate species do not appear in this plot since they constitute less than ∼0.1% of the equilibrium P budget for total P concentrations up to 100 mmolal (SI Appendix, Fig. S13).